Digging into the roots of lupus
White blood cells called neutrophils implicated in the autoimmune disease
Short-lived but populous immune cells called neutrophils and the cellular flotsam they sometimes release might play an important role in lupus. Neutrophils from lupus patients give rise to microscopic nets, made of unwound DNA and proteins, that may trigger the inflammation characteristic of this autoimmune disease, according to two laboratory studies released online March 9 in Science Translational Medicine.
The new work offers “a nice addition to our understanding of the different cellular players that contribute to the immune dysfunction, autoimmunity and inflammation of lupus,” says Mary Crow, a rheumatologist at Weill Cornell Medical College and the Hospital for Special Surgery in New York City, who was not involved in the new studies.
In people with lupus, immune forces attack the body, causing damage to the skin, kidneys, heart, lungs, joints and other tissues. Lupus patients make rogue antibodies that target the body’s own cells, proteins and especially DNA. The result is fatigue, rashes, joint pain and more severe problems, often affecting the kidneys. Inflammation caused by the self-destructive immune reaction underlies these symptoms, with most treatments aimed at quelling that inflammation. Roughly 1.5 million people in the United States have lupus, mostly women in their child-bearing years. Although its cause is unknown, scientists assume a combination of genetic and environmental factors are involved.
Neutrophils, the most abundant white blood cells, have long attracted researchers’ interest. Patients often have a shortage of normal neutrophils in the blood and an excess of immature neutrophils, which get rushed into duty when the supply of mature cells runs low, says Virginia Pascual, a pediatric rheumatologist at the Baylor Institute for Immunology Research in Dallas. But neutrophils’ role in lupus has never been clarified.
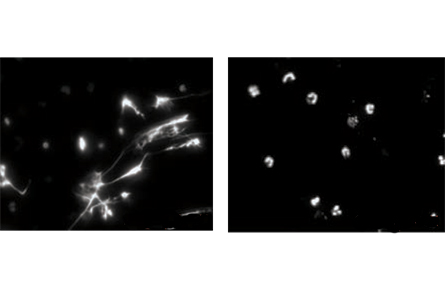
Neutrophils normally serve on the front lines against disease. But in one of the two new studies, Pascual and her colleagues show in lab tests that exposing neutrophils from lupus patients to abnormal antibodies, also from lupus patients, induces the neutrophils to die rapidly in culture. What’s pivotal is how the cells expire: The neutrophils’ components are extruded from the cell and form neutrophil extracellular traps, or NETs, weblike structures that comprise unwound strings of DNA, proteins and other cell components.
When neutrophils from healthy people were exposed to the rogue lupus antibodies, the cells didn’t release their contents in NETs.
Normally, sticky NETs can form in healthy people but serve an immune purpose by snagging bacteria or yeast, Pascual says. But when NETs are made in response to lupus antibodies, the resulting DNA-protein complex activates other immune cells (a kind of dendritic cell) and triggers overproduction of interferon alpha, a potent immune protein. Interferon alpha activates yet more cells in a cascade that the researchers hypothesize can lead to more abnormal antibody production and the inflammation that damages tissues in lupus patients.
“We don’t prove in this paper that it damages kidneys” specifically, Pascual says. “But that fits very well with this model.” Biopsies of lupus patients show that the same dendritic cells produce interferon alpha in kidney tissues.
In the other study, a team led by researchers at the M.D. Anderson Cancer Center in Houston finds a similar role for neutrophils in lupus. That study also elucidates how specific compounds — immune proteins called toll-like receptors and particularly an antimicrobial peptide called LL37 — play central roles in the aberrant chain reactions involving neutrophils. That suggests these compounds might make good targets for scientists seeking to derail this inflammatory cascade.
Crow says, “These two papers place a new emphasis on a role for neutrophils and on NETs — a fairly new concept — as a collection of stimulatory materials that could be responsible for interferon production.”
The two studies push interferon alpha “front and center” in lupus, says Yale physician and immunobiologist Joseph Craft, writing in the same issue of Science Translational Medicine. The reports demonstrate that the release of NETs by neutrophils and their capacity to bind to the dendritic cells “drive the chronic interferon alpha production seen in many patients with lupus.”
Identifying the roles of specific cells and proteins in lupus justifies ongoing efforts to target “the pathogenic steps in disease, perhaps using combination therapies,” Craft says.