Elemental escape
Making superheavies may reveal island of stability
As nuclear physics vacation spots go, the “island of stability” sounds pretty good. But this island isn’t in the Caribbean, the Maldives or even Hawaii. It’s at the edge of the periodic table of the elements.
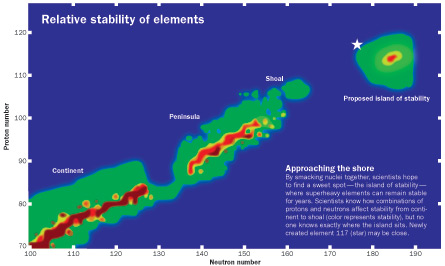
Reaching the island would be the culmination of decades of synthesizing artificial elements, those heavier than uranium (SN: 4/15/78, p. 236). By smashing smaller elements together, researchers have shoved more and more protons and neutrons into a single atomic nucleus. Jam-packed products that include more than 110 to 112 or so protons in each nucleus are generally called “superheavy” elements.
By studying superheavy nuclei, researchers could gain fundamental insights into the nature of matter. But all of the superheavy elements created so far are very unstable, typically decaying into other, lighter-weight elements within fractions of a second.
Theory suggests that if physicists could cram just the right amount of stuff into a nucleus, the resulting element would hit a sweet spot. It could romp on the island of stability for days, months or even years.
When the synthesis of element 117 was announced in April, scientists celebrated it as a sign of getting closer to the island’s shore. But they still haven’t reached dry land. “I would say we’re approaching the island,” says Mark Stoyer, a nuclear physicist at Lawrence Livermore National Laboratory in California who was on the team that made element 117. “We still have a lot more exploring to do.”
Cramming more in
The quest to make superheavy elements takes chemistry to its limits. In lighter-weight elements, protons and neutrons stick together because of the attractive power of the strong nuclear force. But as more and more protons get packed into a nucleus, the strong force begins to be overwhelmed by the Coulomb force, which causes particles of the same charge to repel each other. Within milliseconds, superheavy atoms begin to spit out alpha particles — made of two protons and two neutrons — and shortly thereafter the superheavy nuclei undergo fission, splintering into multiple lighter nuclei.
Overcoming the repulsive Coulomb force requires a couple of tricks, including getting as many neutrons as possible into the nucleus. Being electrically neutral, neutrons help diminish the amount of repulsion pushing protons away from each other.
The Russian-led team that created element 117 used this trick, powering up a calcium nucleus with eight extra neutrons and slamming it into berkelium, which itself had 152 neutrons. The 20 protons in the calcium and 97 protons in the berkelium combined to make an element with 117 protons. The team created two variants, or isotopes, of the new element, one with 177 neutrons and one with 176 neutrons (SN: 4/24/10, p. 15).
Another way to stabilize superheavy elements is to take advantage of the fact that protons and neutrons like to arrange themselves in layers like shells. A completely full shell is extra-stable, and a nucleus that has both filled proton shells and filled neutron shells is said to be “doubly magic.”
But researchers don’t know exactly what combination of protons and neutrons might be most stable in those shells, says Sigurd Hofmann, a nuclear physicist at the GSI research center in Darmstadt, Germany. Some theories predict that the most stable superheavy element would have 114 protons and 184 neutrons; researchers have been able to make an element with 114 protons, but they haven’t succeeded in packing it up with 184 neutrons yet. Other models suggest that proton number 120 or 126, or neutron number 172, might be the ticket.
A further factor in element stability is whether an isotope has an even or odd number of protons and neutrons. For reasons that are not clearly understood, having an odd number slows down the decay process. A nucleus with an odd number of protons plus an odd number of neutrons, says Hofmann, can stick around substantially longer than corresponding even-even ones.
The isotope of element 117 that contains 176 neutrons, for instance, took 21 milliseconds to decay via alpha particle emission to element 115. The isotope with 177 neutrons stuck around longer, for 112 milliseconds. Though the difference might not seem great, for scientists seeking stability it’s substantial.
Approaching dry land
The discovery of element 117 filled the periodic table up to the already-found element 118. The long-lived isotopes of 117 bolster the notion that the island of stability exists, say Hofmann and others. But scientists may not know for sure when they land on its shore.
There is no “sudden island of superheavies,” says theoretical physicist Witold Nazarewicz of the University of Tennessee Knoxville. “This is a gradual transition from nuclei which live for a short time, to those that live for a longer time, to those that will live for years.”
A more philosophical question may be how far chemists can push the concept of synthetic elements. “At some point we’ll reach a point where you can’t have a nucleus bound with so many protons even if you throw in many more neutrons,” Stoyer says. “Is there an end to the periodic table? I don’t think we’re there yet.”
For now, researchers are busy trying to measure the elements that can be made to understand how to get to superheavy shores. One group, led by Michael Block of GSI, reported some progress in February. The researchers captured some atoms of nobelium, atomic number 102, in an electromagnetic trap and measured their mass directly — the first direct mass measurement of an element heavier than uranium. While nobelium itself is not a superheavy element, the improved understanding of its isotopes could “provide reliable anchor points en route to the island of stability,” the team wrote in Nature.
Other experiments will attempt to probe the physical shape of an atomic nucleus. A nucleus with shells full of protons and neutrons should also be spherical, not deformed by the forces trying to tear it apart. So measuring the shape of a nucleus could provide another way to check its stability.
Research hinted in 2006 that scientists can indeed deduce the shape of a nobelium nucleus by studying how it decays away. The work, from teams led by researchers at the University of Jyväskylä in Finland and Argonne National Laboratory in Illinois, was published in Nature and Physical Review Letters.
Some theorists envision pushing superheavy elements to their limits. Nazarewicz, for instance, would like to pack as many as 186 protons into a nucleus, at which point the internal forces would warp the nucleus into a bizarre bubble or doughnut shape. “This is a very theoretical nucleus,” he admits.
While no one should hold their breath for the discovery of element 186, a few new elements may yet be within reach. Current nucleus-smashing techniques could potentially lead to the discovery of elements 119 or 120 within the next couple of years, says Hofmann. Beyond that, researchers will need to find new ways of pumping extra neutrons into target nuclei.
Such efforts will require a new generation of radioactive ion beam facilities to come online, like the Facility for Rare Isotope Beams being built by Michigan State University in East Lansing. Only then can physicists keep expanding the boundaries of the periodic table, hoping to annex the island of stability.