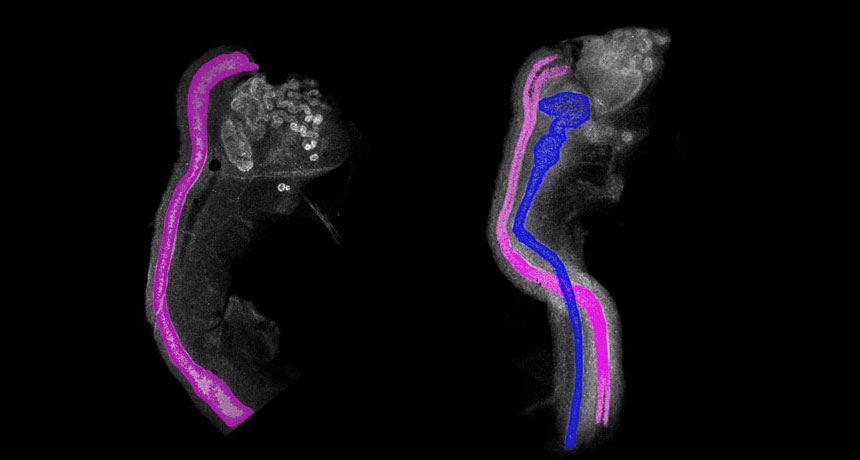
DOUBLING UP A normal female mouse embryo (left) has only female reproductive tissue, called the Müllerian duct (pink). Removing a protein called COUP-TFII causes a female mouse embryo (right) to develop both the female duct and male tissue called the Wolffian duct (blue).
Yao Laboratory/NIEHS