For the first time, scientists have observed how the tiny fungi that live on plant roots begin to break down rocks. The researchers watched fungi disrupt the crystal structure of a common mineral in rocks, physically eroding the rock and also setting the stage for chemical breakdown.
Erosion of bare rock stems from a number of physical, chemical and biological processes. Though scientists generally understand the simple abiotic factors that break down rocks, such as wind and water, the small-scale biological processes that trigger erosion are not as well known.
Scientists have long suspected that ectomycorrhizal fungi — those that live symbiotically on the roots of plants — play a major role in erosion and soil formation, but exactly how these fungi alter minerals was poorly understood, says Steeve Bonneville, a biogeochemist at the University of Leeds in England. So, he and his colleagues set up a lab test to study the nanoscale effects of fungi on biotite, a common potassium-rich mineral found in granite and many other rock types.
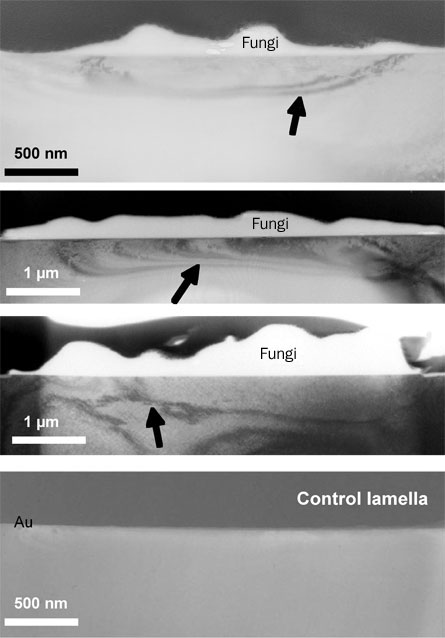
In their test, reported in the July Geology, the researchers planted a pine seedling with Paxillus involutus–covered roots in a dry nutrient-poor soil free of other microorganisms. P. involutus is a common species of ectomycorrhizal fungi. A 1-by-0.5–centimeter flake of biotite inserted near the tree’s roots was the only source of potassium in the soil, Bonneville says. After three months, the researchers used transmission electron microscopy and other techniques to analyze the biotite flake in areas where fungi filaments, known as hyphae, had attached as they grew.
Microscopy revealed that the mineral structure just beneath the filament attachments was disrupted, with mineral layers wedged apart by as much as 14 degrees. Chemical analyses indicate that material near the attachment — as much as 50 nanometers beneath the mineral surface and as much as two micrometers away from the attachment — had lost as much as 70 percent of its potassium, a nutrient that the fungi passed along to the seedling. Parts of the mineral that weren’t attacked by fungi were unaffected, Bonneville notes.
Only after mineral structure had been disrupted by the fungi did chemical changes occur, the researchers report. Wedging apart the crystal structure allowed iron-bearing compounds in the rocks to react with oxygen from the air. This test indicates that erosion can be a two-stage process — with physical changes in mineral structure followed by chemical transformations — and that other soil microbes aren’t necessary, Bonneville and his colleagues contend.
“Without a doubt, a large part of erosion is abiotic,” says Stephen Hillier, a mineralogist at the Macaulay Land-Use Research Institute in Aberdeen, Scotland. Nevertheless, he notes, this is the first time scientists have seen the details of what’s going on below the fungal hyphae that contribute to biotic erosion. “They’ve used a fantastic technique.”