The FDA has canceled emergency use of hydroxychloroquine for COVID-19
The risks of giving the malaria drug don’t outweigh the benefits, the agency rules
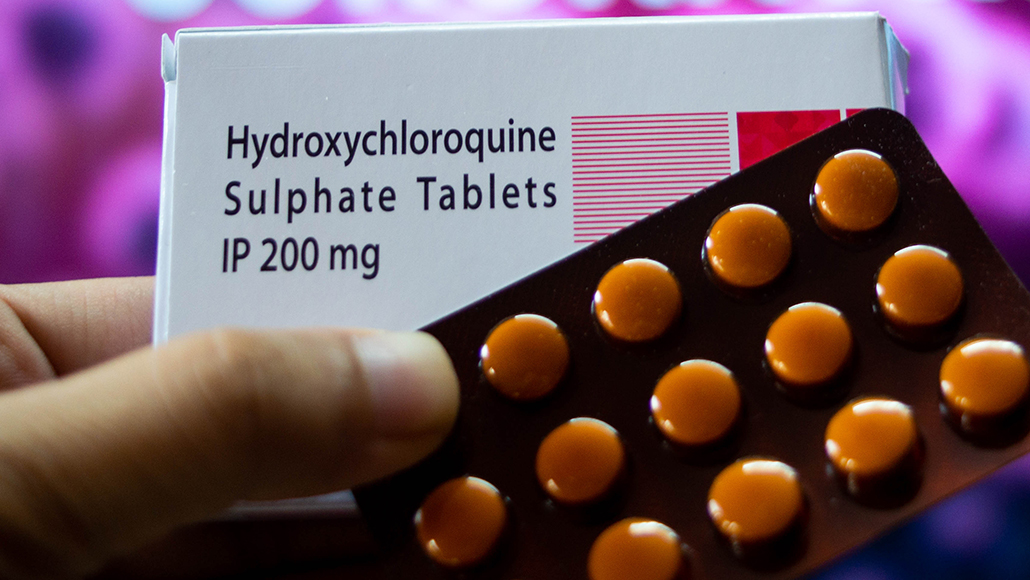
Hydroxychloroquine doesn’t work against the coronavirus, the FDA says. The agency revoked authorization for treating COVID-19 patients with the drug outside of clinical trials.
amlanmathur/iStock/Getty Images Plus