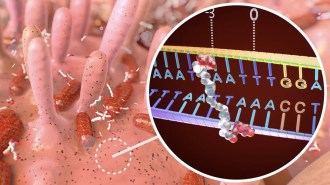
Several pesticides can disrupt a partnership that enables certain plants to take up nitrogen by enlisting the help of bacteria. As well as stunting the growth of those plants, the newfound effect may be decreasing soil fertility, the researchers suggest.

Organisms require nitrogen to make proteins, but most living things can’t use the abundant gas in the atmosphere. A natural process called biological nitrogen fixation converts the gas to ammonia, the form that plants need.
An industrial reaction can do the same trick for crops. Today, farmers apply seven times as much synthetic nitrogen fertilizer as they did 40 years ago. But the higher crop yields that once resulted from fertilizer use have stagnated in recent years, says environmental scientist Jennifer E. Fox of the University of Oregon in Eugene. Moreover, leftover fertilizer leaches into waterways, creating zones choked by algae and uninhabitable by fish, she notes.
Farmers can also replenish soil’s nitrogen stores by growing legumes such as alfalfa and soybeans, which partner with Rhizobium bacteria to fix nitrogen.
Alfalfa teams with the bacterium Sinorhizobium meliloti. The plants send out a chemical signal that binds to a receptor inside S. meliloti, drawing the microbes to the plant. The bacteria set up house in nodules along the roots, where they convert nitrogen gas to ammonia for the alfalfa in exchange for energy.
Fox, John A. McLachlan of Tulane University in New Orleans, and their colleagues had previously found that some pesticides added to cultures of S. meliloti bind to the receptor meant for alfalfa’s signal. In the current study, published in the June 12 Proceedings of the National Academy of Sciences, the team assessed the pesticides’ effects on the alfalfa.
The researchers treated the seeds and their bacterial partners with one of three pesticides: methyl parathion, DDT, and pentachlorophenol. Treatment by each chemical reduced the number of alfalfa-root nodules and decreased alfalfa yields. By 4 weeks after treatment, pentachlorophenol diminished yields the most, to one-sixth the yields from untreated seed and bacteria.
Three alfalfa harvests are typical for a summer season, says Fox. By delaying the plants’ growth, pesticide residues in soil could cut those harvests down to two, “render[ing] legume-crop rotations less effective for maintaining soil fertility,” she and her collaborators say.
The fact that certain pesticides can disrupt the signaling between S. meliloti and alfalfa is “a really interesting and important finding,” says microbial ecologist Donald R. Zak of the University of Michigan in Ann Arbor. However, he notes that it remains to be seen whether the chemical concentrations used in the study typically exist in soil. “What we need to understand is what their concentrations are in alfalfa fields under current management practices,” he says.
The new report “demonstrates the potential for this to be an important process to consider,” says biogeochemist Alan R. Townsend of the University of Colorado at Boulder. But he agrees that the next step is to monitor what happens in the field. “What the roots of the plants are actually seeing” in the soil over time will be key to understanding the strength of the effect, he says.