A fungus weaponized with a spider toxin can kill malaria mosquitoes
In field trials, genetically engineered Metarhizium pingshaense reduced numbers of the insects

MOSQUITO WATCH Entomologist Etienne Bilgo observes a mosquito breeding puddle inside a net-encased structure called the MosquitoSphere. Bilgo is part of a team that tested the ability of a genetically engineered fungus to kill mosquitoes that can spread malaria.
Oliver Zida
A fungus engineered to produce a spider toxin could help take down insecticide-resistant mosquitoes that can spread malaria.
In a netted, outdoor experiment in Burkina Faso, the genetically engineered fungus wiped out mosquito populations within two generations, researchers report in the May 31 Science. If the result holds up in a real-world situation, the modified fungus may one day become a tool for controlling mosquitoes that can transmit the deadly disease.
In 2017, an estimated 219 million people in 87 countries were infected with malaria, and 435,000 died, according to the World Health Organization. Africa carried most of the malaria burden, with 92 percent of cases and 93 percent of deaths occurring on the continent that year.
The fungus Metarhizium pingshaense, long known to infect and kill mosquitoes, was made even deadlier to the insects by the addition of a gene that produces a spider bite toxin called Hybrid. Researchers engineered the fungus to make Hybrid in the presence of the mosquito version of blood, called hemolymph. “We’re just bypassing the spider fangs and getting the fungus to do the same job,” says study coauthor Raymond St. Leger, an entomologist at the University of Maryland in College Park.
In laboratory trials in 2011, engineered fungi related to M. pingshaense infected and killed mosquitoes and their malaria parasites (SN Online: 2/25/11). (The fungi don’t harm people, other insects or animals.) “That’s all well and good, but what happens in the lab doesn’t necessarily translate into field conditions,” says study coauthor Brian Lovett, an insect pathologist and bioengineer also at the University of Maryland.
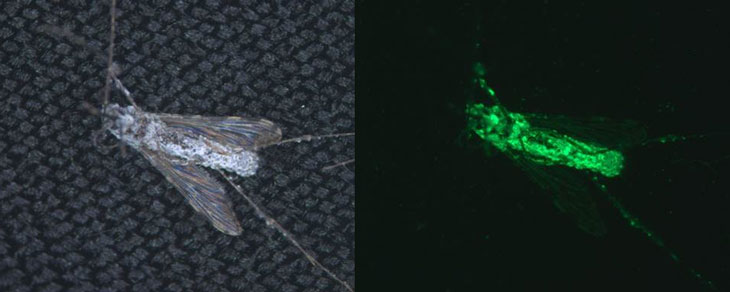
The fungi don’t hold up well in heat or under ultraviolet light and needed to be tested under real-world conditions. But because the engineered M. pingshaense carries a foreign gene, “we can’t just waltz out into the open field and start applying it to people’s houses,” Lovett says.
Working with scientists and villagers in a western region of Burkina Faso where malaria is endemic, the researchers built a giant frame enclosed by two layers of mosquito netting that was divided into sections with huts on the inside. In each hut, a black cloth coated in sesame oil hung on one wall, providing a place for female mosquitoes to rest after feeding. The oil helped fungal spores stick to the cloth. Huts contained a cloth with either no spores, normal spores that don’t make the spider toxin or Hybrid-producing spores.
Each hut was then populated with 1,000 adult male mosquitoes and 500 females, grown from larvae and eggs from insecticide-resistant Anopheles coluzzii collected by local people from puddles. In mating, females dive into a swarm of males; after mating, females must feed on blood to support egg-laying. The mosquitoes drank blood from calves introduced into the huts for three nights each week. The researchers then counted how many adult mosquitoes survived in subsequent generations.
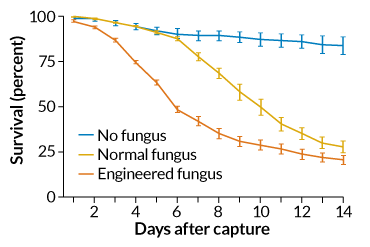
In the hut without the fungus, 921 mosquitoes hatched in the first generation and 1,396 in the second about 25 days later. In the hut with the nonengineered fungus, fewer mosquitoes hatched, with just 436 mosquitoes in the first generation and 455 in the second. That’s a sign that the fungus alone kept numbers down, but didn’t eliminate the insects.
In the hut with the toxin-producing fungus, 399 mosquitoes hatched in the first generation. But in the second generation, only 13 adults made it. That’s not enough mosquitoes to form a mating swarm, so the population was essentially wiped out, Lovett says. The experiment was repeated three times during the rainy season from June to October, each with similar results.
“The results are exciting, but there’s still a lot of work to be done,” says Adriana Costero-Saint Denis, an entomologist at the U.S. National Institute of Allergy and Infectious Diseases in Rockville, Md, which funded part of the work. The researchers still need to work out variables, including where best to hang the cloth — from the ceiling or on walls, in bedrooms or near doors and windows, she says. And while the study “was a contained, artificial environment,” she says, “temperature and humidity were natural, so it’s better than the lab.”
If the engineered fungi prove useful in future tests, they might be combined with insecticides or other mosquito control measures to tackle malaria, says medical entomologist Nsa Dada. She is affiliated with the U.S. Centers for Disease Control and Prevention in Atlanta but spoke about the study in a personal capacity. Dada wants to know if the fungus is equally effective against other mosquito species that can carry malaria.
While supporting the development of new anti-mosquito tools, ecological entomologist Matthew Thomas says the study still doesn’t establish whether the engineered fungus is an improvement over nature.
The genetically modified fungus cut the study’s mosquito populations to about 25 percent of the starting number in 14 days, while other species of unaltered fungi can kill 100 percent of mosquitoes in five or six days, says Thomas, of Penn State University. “So it’s not clear what benefit this has provided,” he says. “It’s almost like a technology looking for an application, rather than a problem needing fixing.”