Whirring softly, the motorized ring of a scanner spins around a brain cancer patient’s head in a New York City hospital. An X-ray tube mounted in the doughnut-shaped device emits pulses of high-energy radiation that travel unfelt through the person’s skull as the patient glides deeper into the scanning machine. Similar scenes occur daily at thousands of hospitals in which computerized tomography, or CT scanning, has become a mainstay of medical diagnosis. It may not be fun, but it’s less risky and painful than surgery for finding out what’s going on inside a patient.
For this medical application, researchers have developed a complex set of mathematical tools to take information collected from X rays and convert it into images of the body’s internal structures. Now, scientists in Canada and Japan have shrunk such technology to the molecular scale.
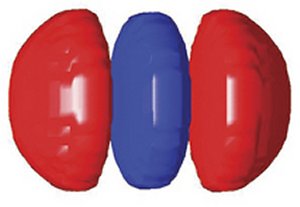
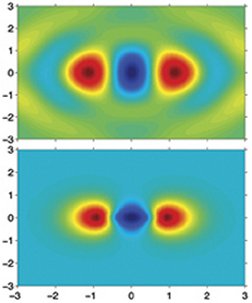
Instead of patients in medical gowns, the CT-scan subjects of these scientists are puffs of nitrogen gas released into a vacuum chamber. Rather than X rays, the source of information is radiation emitted by an exquisitely fast vibration of electric charge within the molecule. In place of imaging human tissue layer by layer, as a medical CT scan does, the scientists assemble images of slivers of molecules into detailed portraits that, for the first time, depict the spatial distribution—the quantum actions—of the molecules’ outermost electrons.
Call it quantum CT scanning.
As one of the latest strides in observing the cloudlike envelopes of electrons within atoms and molecules, the technique raises the prospect that scientists may soon watch the shifting shapes of such clouds as bonds break and form in chemical reactions.
“This would be progress indeed and provide insight into one of the most fundamental steps in chemistry,” comments Henrik Stapelfeldt of the University of Aarhus in Denmark.
Racing pulse
In the quantum realm, matter stands still for no one. Electrons and other particles smear out as diffuse—albeit tiny—clouds of matter and charge. The electron clouds, which scientists call orbitals, morph into different shapes at staggering speeds. The configuration can change many millions of billions times per second. Scientists plan to use quantum CT scans to observe such split-second transformations, during which chemical bonds are broken and formed.
In the past 2 decades, chemists and physicists have made great leaps toward visualizing those chemical transformations. Much of that progress has depended on lasers. As optical scientists and engineers have learned to cram ever-more-intense bursts of light into ever-more-narrow slices of time, they’ve opened ever-clearer windows on the chemical activity of atoms and molecules.
It’s like using strobe lights in high-speed photography to capture the wing of a hummingbird in midbeat or a drop of milk at the moment of impact, says Thomas C. Weinacht of the State University of New York at Stony Brook. The briefer the light pulse, he notes, the faster the process that can be probed, prodded, and recorded.
Lasers can now routinely generate pulses lasting just millionths or billionths of a second, or femtoseconds, so scientists have begun to produce what amount to snapshots of fleeting, intermediate stages of chemical reactions. By doing so, they’ve identified previously unobserved combinations and fragments of reactants that form during the course of a reaction along the way to the final products.
Taking further advantage of extra-short bursts of laser light, David M. Villeneuve of the National Research Council of Canada in Ottawa and his colleagues devised quantum CT scanning. It takes pictures at unprecedented speeds without immediately disrupting a molecule’s electron clouds.
This approach contrasts with other applications of short laser pulses to molecules. Some researchers have used these blazingly fast pulses to force electron clouds into specific configurations. For example, Weinacht and his colleagues zap molecules with laser pulses crafted to favor specific chemical products in reactions that, on their own, might yield different products.
Little patients
In a medical CT scan, X rays pass through tissue at a series of different angles, and a computer analyzes the X rays’ subsequent locations and intensities to generate images of thin slices of the body. The computer then combines the data on a multitude of slices to produce a 3-dimensional view of inner anatomy.
Villeneuve’s team has found that the same principle works on the molecular scale. There, molecules respond to extremely brief laser pulses by emitting low-energy X rays, also called extreme ultraviolet radiation, into the scanner’s detectors.
The scientists have applied the technique to visualize a nitrogen molecule’s outermost electron orbital—the one that is most available for chemical reactions and therefore has the greatest influence on the molecule’s chemical personality.
“We use the laser field to make a mini [electron] accelerator only a few 10s to 100 angstroms in size. It’s a nanoaccelerator” for probing the interior of atoms and molecules, says Paul B. Corkum, also of the National Research Council.
Quantum CT scanning requires a double shot of laser pulses. The first flash rotates the molecules to a specific angle for the scanner. The second shot then sets off a remarkable quantum process.
The powerful electric field of the laser shot yanks a portion of the outermost electron cloud out of the molecule and then forcefully shoves it back in again. The perturbed piece of orbital interferes with the unperturbed portion.
The interference generates an ultrarapid vibration of electric charge within each molecule. That leads to the emission of high-frequency radiation that carries information about the molecule’s outermost electron’s spatial distribution, as viewed from one angle. By the end of the 30-femtosecond shot the molecule is shattered, but the technique has already collected its information.
To image nitrogen’s outermost electron orbital, the scientists rotated and shot bunches of nitrogen molecules from 19 different angles, each 5 degrees apart. Gathering enough data to image each slice required about 500 runs, says Dirk Zeidler of Villeneuve’s team. Once images of all the slices were in hand, a computer cinched them into a 3-dimensional portrait of the entire cloud.
The group described its new technique in the Dec. 16, 2004 Nature.
Catch a wave
The new molecular-imaging technique relies on laser-light pulses whose associated electric fields change direction within about a femtosecond, notes Robert R. Jones of the University of Virginia in Charlottesville. The influence of that field on a molecule comes and goes so quickly that most of the molecule doesn’t respond.
“It’s like pulling a tablecloth out from under dishes on a table. If you pull it away fast enough, they stay where they are,” Jones says.
The Canadian scientists claim that their new method of probing molecular structure has yielded the most revealing image so far of an electron’s wave function. This function depicts both the amplitude and direction of an electron wave’s oscillations at every point in space.
That’s critical information for understanding properties of atoms and molecules, such as their responses to radiation and readiness to chemically bond, comments Jonathan Marangos of Imperial College London. “It’s sort of an axiom of quantum mechanics that the wave function can tell you absolutely all you want to know about a [quantum] system,” he adds.
What’s particularly promising, says Villeneuve, is that the new approach can “isolate one electron in this sea of electrons [surrounding the atomic nuclei] that are boiling all over the place.” That unprecedented selectivity is vital to the team’s plans to observe changes in molecular electron clouds that are the key to chemical reactions.
“This development marks a new epoch in our … understanding of chemistry” at the finest scales of space and time, says physicist Jonathan G. Underwood of Open University in Milton Keynes, England.
There is still a way to go, acknowledges Corkum. With only two atoms, nitrogen is one of the simplest molecules. No one yet knows if the technique will work with more-complex molecules.
Observing electron clouds in bigger molecules might reveal details of atomic bonds that determine molecular structures, Villeneuve says. However, the orbital wave functions in those structures may be so sprawling and complex that perturbing them with a laser may yield emissions so tainted with extraneous information that scientists won’t be able to make sense of them. Or the laser pulses might fiddle with more than one electron orbital at a time, also muddying the image beyond recognition, says Denmark’s Stapelfeldt.
None of this is stopping Corkum from thinking as big as he can about the molecular realm. In principle, he says, the technique should handle orbitals even the megamolecules of life, such as proteins and DNA. “That would be a dream, but it’s a long-distance dream for now,” Corkum says. “There may be many practical things to keep us from getting there.”
Still, he looks forward to the challenge of peering into molecules that have many thousands of atoms, each with its own entourage of electrons.
Old-Fashioned Atoms
With help, matter’s wispy building blocks turn solid and certain
While some scientists develop new tools to visualize for the first time the fuzzy essence of electrons, others are coercing these inherently quantum entities into clearly definable locations and behaviors that resemble those of tiny billiard balls. The reshaped atoms call to mind an antiquated version of atomic structure that dates to the early 20th century. In this classical view, atoms resemble miniature solar systems in which electrons orbit the nucleus as discrete planets circle a star. With the new research, scientists are exploring how matter crosses the line between quantum and classical forms.
In a recent investigation, researchers in Virginia have used laser pulses and beams of microwaves to take the outermost electron of lithium atoms, generally considered to have a cloudlike, everywhere-and-nowhere character and corral it as a sharply defined, orbiting entity.
Nonetheless, these seemingly classical atoms remain indisputably quantum objects, says Thomas F. Gallagher of the University of Virginia in Charlottesville, who led the experimental team.
Gallagher and his colleagues directed a series of laser pulses into a jet of lithium atoms streaming from a hot laboratory oven. The outermost electrons of the atoms absorbed the energy and assumed ever-larger orbital distances from the atoms’ nuclei. These bloated atoms are known as Rydberg atoms. Then, using microwaves, the scientists made the perked-up electrons take on more-organized motions, thereby behaving more like discrete particles than like fuzzy clouds. Physicists refer to such particlelike quantum entities as wavepackets. The scientists outline their technique in the March 18 Science.
Electrons resemble clouds because the wavelike oscillations of an electron are composed of many vibrations, each of which is characterized by its own frequency, Gallagher says. Altogether, these vibrations define a complex, spread-out geometry.
Under the influence of microwaves tuned to the frequency range of those electron waves, however, that cacophony of vibrations can be organized into a harmony. As a result, the electron—at least for the few microseconds during which a researcher targets it with the microwave bath—coalesces into a discrete entity. This transformation opens the path both to in-depth studies of the quixotic classical-quantum boundary and to new forms of control over atoms, Gallagher says.
By ramping up and down the microwave frequencies, the researchers dialed in the orbital distance and velocity of the electron. “It’s like turning up [or down] the speed of a merry-go-round,” Gallagher says.
In this way, the team controlled the energy binding the electrons to the lithium atom’s nucleus. Binding energies determine an atom’s stability, so the technique can alter the fundamental personality of atoms.
This quantum-to-classical sleight of hand may have practical as well as theoretical payoffs. It could provide a means of tuning atoms so that they respond to single photons of light in a difficult-to-measure terahertz portion of the electromagnetic spectrum, Gallagher says. This could be particularly useful to astronomers for detecting radiation from the early universe, to doctors searching for cancerous tissue in the body, and to security screeners looking for weapons and contraband in packages and under clothing.







