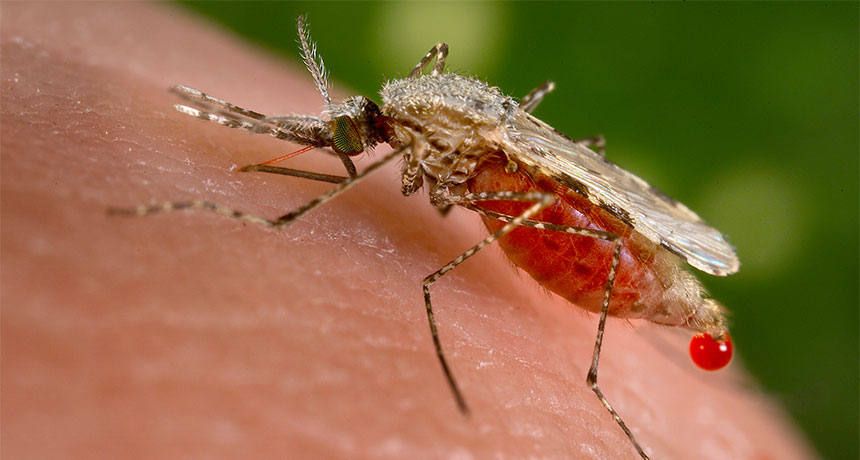
BLOCKING MALARIA Anopheles stephensi (pictured) is responsible for about 12 percent of malaria cases in India. Researchers have now engineered these mosquitoes to spread malaria resistance instead of disease.
Jim Gathany/CDC
A new genetic engineering technique may quickly inoculate mosquitoes against malaria, helping to end the spread of the disease in humans.
Using a gene-editing tool known as CRISPR/Cas9, researchers have made a “genetic vaccine” that will continually inject itself into mosquitoes’ DNA. Such a vaccine, known as a gene drive, could spread to nearly every mosquito in a population within a few generations. The accomplishment, described online November 23 in the Proceedings of the National Academy of Sciences, brings researchers one step closer to malaria eradication.
“This work suggests that we’re a hop, skip and jump away from actual gene drive candidates for eventual release,” says Kevin Esvelt, a synthetic biologist at Harvard University who was not involved in the work.
Gene drives are engineered pieces of DNA that copy and paste themselves into precise locations in an organism’s genome. During mating, a gene drive inherited from one parent inserts itself into the gene inherited from the other parent, ensuring that future generations will almost always inherit the drive. Normally, a gene has a 50 percent chance of being passed to offspring.
CRISPR/Cas9 is a popular new gene-editing system. The editor comes in two parts: a DNA-cutting enzyme called Cas9, and a strand of RNA that guides the enzyme to the location in the genome to be cut. Earlier this year, Valentino Gantz and Ethan Bier of the University of California, San Diego turned the CRISPR editor into a gene drive in fruit flies. That gene drive consisted of DNA containing genes encoding the enzyme and the guide RNA, and allows editing in every generation to perpetuity.
Gantz and Bier teamed up with Anthony James of the University of California, Irvine to build a gene drive in Anopheles stephensi mosquitoes. James and colleagues had previously engineered mosquitoes to make antibodies against malaria. “We’d already built the cargo,” the antibody genes, says James. “They had a drive system and wanted to see if it could carry our cargo.”
It was a lot of cargo to carry. In addition to the antibody genes and those encoding the Cas9 enzyme and guide RNA, researchers inserted a gene that would cause mosquitoes’ eyes to glow red under fluorescent lights, enabling researchers to easily find insects of interest. Getting such a big piece of DNA into the mosquitoes proved difficult; only two of 25,712 larvae screened had the glowing red eyes that indicated they carried the gene drive.
Once in these two mosquitoes, though, the gene drive moved efficiently. In the third generation, 98.9 percent of the progeny of male mosquitoes descended from the original two males carried the gene drive.
That efficiency “is a really good indication that this technology is worth pursuing,” says molecular biologist Zach Adelman of Virginia Tech University in Blacksburg.
But the new drive got a flat tire in female mosquitoes. In most of the offspring descended from daughters of the original male mosquitoes, eye color indicated that Cas9 cut the target DNA but didn’t complete the delivery.
The researchers had engineered the drive to insert itself into the kynurenine hydroxylase, or kh, gene in the mosquitoes. If the drive inserted its cargo as planned, the mosquitoes would have dark, glowing eyes. But if the Cas9 enzyme cut the gene but failed to deliver the cargo, the mosquito would glue the cut ends back together, introducing a mutation that would turn the eyes white. All but three of the 1,781 descendants from the females had white eyes or eyes with white patches.
The mutation also has the effect of eliminating the cutting site for the Cas9 enzyme, making future deliveries of the gene drive cargo to that location impossible.
Female mosquitoes may be loading their eggs with Cas9 so that when sperm fertilize the egg, the kh gene is cut and glued back together before the cargo can be delivered, the researchers say. That would eventually make the drive useless in wild populations, James says. He and colleagues are working to devise a new drive that would be active only in males.
Gene drives alone won’t eliminate malaria, but could help clear the disease and keep it from coming back, James says. Current malaria eradication efforts are often thwarted when disease-carrying mosquitoes migrate into an area where mosquitoes have been killed with insecticides or other measures. Gene drive‒carrying mosquitoes could breed with interlopers and inoculate them against malaria, holding the scourge at bay.







