Multiple sclerosis has a common viral culprit, opening doors to new approaches
Researchers can now pivot to vaccines and new treatments

Epstein-Barr viruses (red) emerge from an immune system B cell in this colorized electron micrograph.
STEVE GSCHMEISSNER/SCIENCE SOURCE
As Tanina Agosto went through her normal morning routine in July 2007, she realized something was wrong. The 29-year-old couldn’t control her left side, even her face. “Literally the top of my head to the bottom of my foot on the left side of my body could not feel anything.”
The next day, Agosto spoke with a doctor at the New York City hospital where she works as a medical secretary. He told her that she probably had a pinched nerve and to see a chiropractor.
But chiropractic care didn’t help. Months later, Agosto needed a cane to get around, and moving her left leg and arm required lots of concentration. She couldn’t work. Numbness and tingling made cooking and cleaning difficult. It felt a bit like looping a rubber band tightly around a finger until it loses sensation, Agosto says. Once the rubber band comes off, the finger tingles for a bit. But for her, the tingling wouldn’t stop.
Finally, she recalls, one chiropractor told her, “I’m not too big of a person to say there’s something very wrong with you, and I don’t know what it is. You need to see a neurologist.” In November 2008, tests confirmed that Agosto had multiple sclerosis. Her immune system was attacking her brain and spinal cord.
Agosto knew nothing about MS except that a friend of her mother’s had it. “At the time, I was like, there’s no way I’ve got this old lady’s condition,” she says. “To be hit with that and know that there’s no cure — that was just devastating.”
Why people develop the autoimmune disorder has been a long-standing question. Studies have pointed to certain gene variations and environmental factors. For decades, a common virus called Epstein-Barr virus has also been high on the list of culprits.
Now recent studies paint a clearer picture that Epstein-Barr virus instigates MS when the central nervous system gets caught in the cross hairs of an immune response to the virus’s attack. This recognition opens new options for treatment, or even vaccines. Perhaps therapies that target Epstein-Barr itself — or remove the cells in the body where the virus camps out — could jettison the virus before damage is done.

Vaccines might one day “make multiple sclerosis become a historical disease like polio,” says Lawrence Steinman, a neurologist at Stanford University. “The trials will be arduous,” Steinman says. Still, “I think we might be able to put MS in the rearview mirror.”
For now, there’s a lot to learn, including how exactly the virus triggers MS, says Francesca Aloisi, a neuroscientist at the Italian National Institute of Health in Rome.
For many people with MS, even with current therapies, the disease can progress. Right now, Agosto’s symptoms are largely under control. Thanks to physical therapy, an anti-inflammatory diet and medication, she has about 90 percent function on the left side of her body. “Things like long-distance running are out of the question,” she says. Carrying grocery bags with her left arm is a challenge.
Studying the virus’s role in MS “will be an amazing game changer,” says Agosto, who is a patient advocate with the National Multiple Sclerosis Society’s New York City chapter. If Epstein-Barr virus is driving her disease, she wants to know: “How do we get this virus out of the driver’s seat?”
A familiar virus
Multiple sclerosis is an uncommon disease, affecting nearly 3 million people globally. Yet Epstein-Barr virus is almost everywhere.
The virus, discovered in 1964, infects an estimated 90 percent of people around the world. People infected as young children might have a mild cold or show no symptoms. Teenagers or young adults may experience a bout of debilitating fatigue called infectious mononucleosis, or mono, that can last weeks or months.
These symptoms eventually fade. But Epstein-Barr infections hang on. The virus belongs to the herpesvirus family — a group known for instigating lifelong infections. The herpesviruses behind cold sores, genital herpes and chicken pox also stick around for life, usually staying quiet for long stretches. For example, varicella-zoster virus, which causes chicken pox, goes latent inside nerve cells but can resurface to cause the painful disease shingles (SN: 3/2/19, p. 22).
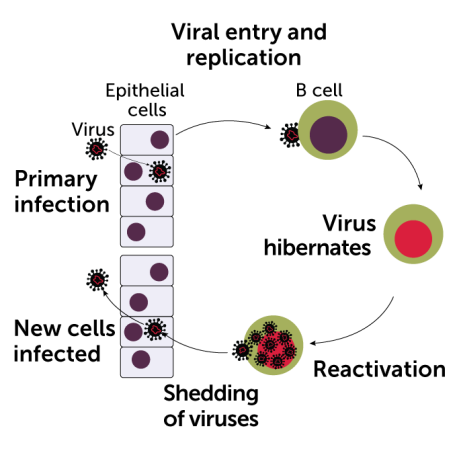
In the body, Epstein-Barr virus slips into the epithelial cells that line the surface of the throat, allowing the virus to spread to other people via saliva — hence mono’s nickname, “the kissing disease.” The virus also infects a type of immune cell called B cells, where it enters viral hibernation.
Epstein-Barr virus can cause problems long after the initial infection. People who had mono are more likely to develop cancers such as Hodgkin’s lymphoma than people who didn’t. And they are more likely to be diagnosed with MS. A teenage case of mono doesn’t mean long-term problems are inevitable. But avoiding mono-related fatigue doesn’t guarantee an escape from risk either. Agosto, for instance, doesn’t recall ever having mono.
Establishing the link
In March 2000, epidemiologist Alberto Ascherio of the Harvard T.H. Chan School of Public Health published research exploring the link between Epstein-Barr virus and MS. With colleague Mette Munch of the University of Aarhus in Denmark, Ascherio analyzed data from eight studies suggesting that MS patients are more likely to have had an Epstein-Barr infection than those without MS. Studies over the next 20 years continued to hint that the virus plays a role, but “the problem is to go from a suggestion or suspicion to proof,” says Ascherio. Getting that proof is difficult, because nearly everyone has been infected with Epstein-Barr virus, or EBV, yet very few have MS.
“If it’s true that EBV causes MS, then you would expect to find that those individuals who are not infected with EBV, they will not get MS,” Ascherio says. “It’s very simple.” He and colleagues needed to follow a large group of young adults who had never been infected.
The researchers found such a group in the U.S. military. Through the Department of Defense Serum Repository, the team had access to repeated blood samples from more than 10 million individuals, taken when active-duty members were screened for diseases such as HIV at the start of their service and then every two years. Using blood samples taken between 1993 and 2013, Ascherio and colleagues could identify people who had never been infected with Epstein-Barr virus, track new infections and learn when people who developed MS started showing symptoms.
Over that 20-year span, 801 people whose blood was tested were diagnosed with MS. Thirty-five of those people had no signs of Epstein-Barr virus infection in their first blood sample. But all but one became infected before their MS diagnosis. People infected with the virus were 32 times as likely to develop MS as uninfected people. What’s more, the researchers found that blood concentrations of a nervous system protein that is a signal of nerve damage rose after Epstein-Barr virus infection, before an MS diagnosis. The results prompted Ascherio and his team to make a bold claim in Science in January: “These findings cannot be explained by any known risk factor for MS and suggest EBV as the leading cause of MS.”
It is still possible that infection with Epstein-Barr virus is a time stamp for something else, perhaps not yet identified, that’s also relevant for MS, says Mark Allegretta, vice president of research at the National Multiple Sclerosis Society. “The way we talk about it now is that it’s very strong evidence that it’s necessary for development of MS, but it’s insufficient on its own.”
Ascherio isn’t deterred. “After 20 years of talking about EBV and MS, it’s quite exciting that we’ve finally nailed it down,” he says. “There was a lot of skepticism until now and that is fading away.”
A skeptic convinced
The fact that Epstein-Barr virus is implicated in so many diseases had many researchers skeptical of its link to MS, says Tobias Lanz, a neurologist at Stanford University. “It’s involved in tumors, it’s involved in MS, it’s involved in lupus, it’s in chronic fatigue syndrome. Somehow, people link it to everything and that makes us reasonably suspicious.”
Lanz’s mentor, Stanford rheumatologist William Robinson, was one of those skeptics. Once Lanz, Robinson and their colleagues found hints of how Epstein-Barr virus could spark nerve damage, however, Robinson became a believer.
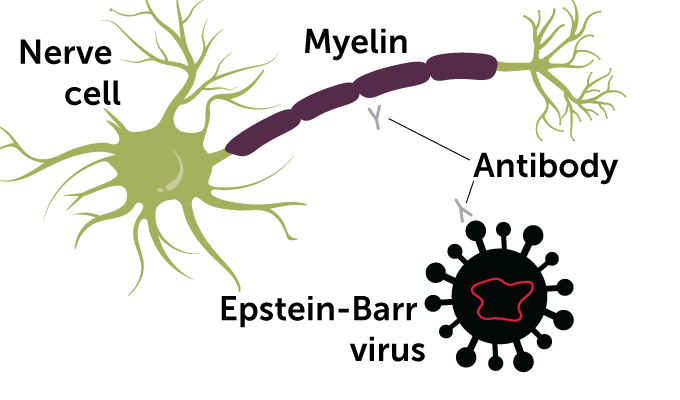
The team discovered that immune proteins called antibodies from some MS patients attach to a key Epstein-Barr virus protein, as well as to a protein from the central nervous system. This finding, described in the March 10 Nature, suggests that as the immune system learns how to recognize the virus, it may also learn to attack nerve cells.
The viral protein, called EBNA1, helps Epstein-Barr virus persist in the body for life, hidden away inside B cells. Its molecular twin in the central nervous system, a portion of a protein called GlialCAM, is so similar that antibodies for the virus recognize and bind tightly to it too, the team found in lab experiments.
“That really changed everything,” Robinson says, calling it “an in-your-face result that you can’t dismiss as not being real.” In addition to adding to the evidence that Epstein-Barr virus causes MS, the finding also provides a hint of a possible mechanism: GlialCAM is found in glial cells, which support nerve cells and form the insulating layer myelin that helps nerve cells send signals (SN: 8/22/15, p. 18). Myelin is the very thing that is destroyed in MS.
About a quarter of patients in the study had antibodies that recognize both EBNA1 and GlialCAM. The similarities between the two proteins, called molecular mimicry, means that EBNA1 may not be a good viral protein to include in vaccines to curb diseases related to Epstein-Barr virus, says Steinman, the Stanford neurologist, who was also involved with the research. If the virus indeed sparks an autoimmune reaction, vaccines that target this viral protein or other mimics could harm myelin and spur MS.
Viral damage
Several studies support the idea that molecular mimicry causes MS damage. But other hypotheses are on the table.
Those B cells, for instance, where Epstein-Barr viruses hide out, produce antibodies. One possibility is that B cells infected with Epstein-Barr virus transform in ways that encourage the immune system to attack the body’s own tissues.
Aloisi, the neuroscientist in Rome, backs a different hypothesis: Perhaps the immune system’s attack on the virus itself is behind the damage.
“The biology of the virus is so similar to the biology of the disease,” Aloisi says. For some people, MS can go through phases of silence where the disease is stable, no better, no worse. The disease then reactivates, producing new brain lesions and worsening symptoms. Epstein-Barr virus can similarly come out of latency, perhaps causing a surge of problems before returning to hibernation inside host cells.
In 2007, Aloisi and colleagues discovered unexpected clusters of B cells within the membranes that cover and protect the brain. In all but one of 22 patients studied, some of those B cells were infected with Epstein-Barr virus.
The finding “was like a bomb in the field,” Aloisi says, “because nobody ever thought about this possibility.” Other researchers initially failed to replicate the results. But “little by little other work came out [in support],” she says. “It’s difficult to find these [clusters of B cells] in the brain because people with MS don’t have large, inflamed brains. It’s small spots here and there.”
It’s possible that the central nervous system becomes a stronghold for the virus, Aloisi says. Immune cells called T cells, which can either coordinate an attack or kill infected cells, rush in. Some virus-infected B cells die, but the immune system can’t eliminate the virus. Myelin gets caught in the cross fire. “This creates a situation that is extremely detrimental to the tissue,” she says.
We summarize the week's scientific breakthroughs every Thursday.
Treatment tactics
Regardless of whether Epstein-Barr virus drives MS symptoms directly or causes the body’s immune response to go haywire, the big question is what to do about it.
One obvious path is to develop MS drugs that go after the virus, Aloisi says. Some drugs that block hepatitis B virus and HIV have shown potential against Epstein-Barr virus in lab-grown cells, says Ascherio, the Harvard epidemiologist. But those results are very preliminary.
Another option is to go after the infected cells. A few MS therapies may do that already. The existing MS therapy natalizumab already prevents B and T cells from crossing into the central nervous system. Fingolimod may do that as well. Another drug called ocrelizumab, approved for patients with MS in 2017, is an antibody that attaches to a protein on B cells and triggers cell death. The drug helps patients, like Agosto, who have relapsing-remitting MS, but it’s less effective for people with a progressive form of the disease, who have fewer treatment options (SN: 12/9/17, p. 20).
Researchers thought the drug dampened faulty immune responses by depleting B cells, Lanz says. “But it could also well be that we’re hitting those particular pathogenic B cells that are infected with Epstein-Barr virus. So the B cell depletion might actually be an anti-EBV drug and nobody appreciated that.”
Aloisi agrees. “Now we need something that targets the EBV-infected cells, not all of the B cells,” she says. Indiscriminately killing B cells puts patients at risk for other infections. One way to get around that could come in the form of T cell therapies that go after only infected cells. Such therapies are already in clinical trials in MS patients.
Some researchers suspect that antiviral treatments would probably make the most sense when used early on, before the immune system eats away at the myelin around the nerve cells. Once the virus has kick-started an immune response to attack the nervous system, “the train may already be out of the station,” says neuroimmunologist Emily Harrington of Ohio State University’s Wexner Medical Center in Columbus.
A vaccine
Even better than stopping the infection once it starts would be to build defenses before the virus invades, or to stop it from reawakening. Enter vaccines.
The widespread impact of mono and Epstein-Barr virus’s links to cancer and autoimmune disease had already spurred vaccine research, so a few potential shots are already in the pipeline. But Epstein-Barr virus has a complex way of invading the body, says vaccinologist Javier Gordon Ogembo of City of Hope, a cancer care center in Duarte, Calif. The virus uses at least five viral proteins to invade both epithelial cells and B cells. A vaccine would need to drive an immune response that blocks the virus’s entry into both cell types to prevent infection. “This is the reason, I think, why there has not been a vaccine so far,” Ogembo says.
Pharmaceutical company GlaxoSmithKline took one vaccine candidate to clinical trials in the early 2000s. It seemed to stop people from developing mono, but it didn’t meet the original goal of preventing infection overall. So the company abandoned the vaccine.
Moderna, the biotechnology company made famous for its effective COVID-19 vaccine, recently launched a clinical trial of an mRNA vaccine for Epstein-Barr virus. The shot teaches the body to recognize four of the five viral proteins that help the virus invade both cell types, says viral immunologist Katherine Luzuriaga of the University of Massachusetts Chan Medical School in Worcester, who is involved in the trial. For now, the team is testing whether the vaccine sparks a strong immune response and getting a sense for whether it might curb cases of mono.
In March, the U.S National Institutes of Health launched a clinical trial to test a vaccine that uses nanoparticles to teach the body to recognize the virus and get rid of it. Ogembo and colleagues at City of Hope are developing another vaccine that uses a modified virus as the immune system’s instructor.
Although clinical trials could reveal within the next few years whether the vaccines can control mono, it will be decades before researchers learn anything about the potential impact on cancer or MS, Luzuriaga and Ogembo say. The hope is to see an outcome like the vaccines for human papillomaviruses, Luzuriaga says, which reduce the number of HPV infections and led to a dramatic reduction in cervical cancers.
Developing therapeutic vaccines for people who already have MS may also be possible, Ascherio says. The aim would be to stop the virus from emerging from its slumber inside B cells. It would be akin to the shingles vaccine, which prevents the painful reactivation of varicella-zoster virus in nerve cells.
That is Steinman’s aim as well, but he envisions a shot that would put a check on the undesirable immune response. Steinman and colleagues have tested such a vaccine to try and teach MS patients’ immune systems to ignore and not harm a protein called myelin basic protein, which helps add myelin to nerves. There were hints the vaccine might have been effective, but the team ultimately stopped the project.
“If it weren’t for other very powerful therapies becoming approved in that same time frame, we may have continued,” Steinman says. Now, he wants to make a vaccine that helps MS patients tolerate, rather than attack, the central nervous system protein GlialCAM.
Researchers at BioNTech, also famous for developing a COVID-19 vaccine, are working on something similar. In mice with a disease close to MS, the company showed that an mRNA vaccine could keep the immune system from attacking myelin proteins, the team reported in January 2021 in Science.
Time will tell how effective any of these shots might be. But with studies providing more and more evidence that Epstein-Barr virus is linked to many diseases, Ogembo says, “it’s time to make a vaccine and get rid of it.”