A newly approved drug could be a boon for treating malaria
But people with a certain genetic mutation who use tafenoquine could be at risk of anemia
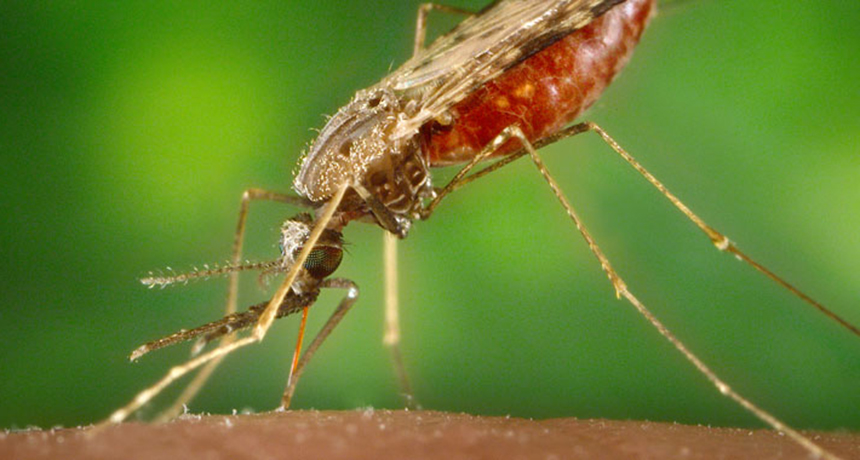
STEALTHY SUCKER This Anopheles albimanus mosquito is one of several species that can transmit the recurring form of malaria. A new drug could help stem such relapses.
CDC Global/Flickr (CC By 2.0)
The first new treatment in 60 years for a particularly stubborn kind of malaria is raising hopes that it might help eradicate the disease, even though the treatment can cause a dangerous side effect.
Called tafenoquine, the drug targets the parasite that causes relapsing malaria. Plasmodium vivax infects an estimated 8.5 million people, mainly in Asia and Latin America. Each time infected people have a malaria relapse, the parasite returns to their bloodstream, allowing them to transmit the infection if a mosquito bites them again. Tafenoquine was approved as a treatment in July by the U.S. Food and Drug Administration and is under consideration as a preventative medication, too.
“This is a game changer because we’ve really been struggling with eliminating [P.] vivax,” says malaria physician Ric Price from the Menzies School of Health Research in Darwin, Australia.
The FDA’s action is expected to spur other countries where relapsing malaria is more prevalent to approve the drug as well. Companies are also working to develop speedy, low-cost tests that can identify people with a genetic deficiency who may risk getting a kind of anemia from the new drug. This test is essential for putting the drug to use in rural areas where rates of both P. vivax and this deficiency can be high.
Like its deadlier cousin P. falciparum, P. vivax is spread by the Anopheles mosquito and causes chills, a cyclical fever and joint aches (SN: 3/18/17, p. 10). Unlike P. falciparum, once inside the body, P. vivax can stay dormant in the liver for weeks or months before flaring up again and again.
Tafenoquine (which will be marketed in the United States as Krintafel) is designed to prevent these relapses. It is similar to an older drug called primaquine, but it is taken in two 150-milligram doses a few hours apart, instead of daily for two weeks. In clinical trials, that dosage, paired with acute malaria medication chloroquine, prevented 70 percent of malaria relapses.
With its compressed dosing schedule, patients prescribed tafenoquine are more likely than those on primaquine to complete the treatment, preventing more relapses and problems with drug resistance.
But tafenoquine’s biggest selling point is also a weakness. Both primaquine and tafenoquine can cause a dangerous side effect in people with glucose-6-phosphate dehydrogenase, or G6PD, deficiency, an abnormality on the X chromosome that affects 400 million people worldwide. When people who have G6PD deficiency take these medications, they are at a higher risk for hemolytic anemia, the destruction of red blood cells.
Tafenoquine’s long-lasting formula makes it harder for the body to clear it out, which could potentially lead to life-threatening damage to red blood cells and severe anemia. “Once it’s in your body, it stays in your body,” says Nick White, a physician and malaria expert at the Mahidol Oxford Tropical Medicine Research Unit at the University of Oxford in Bangkok.
Tests to determine which patients have G6PD deficiency are not widely available, particularly in developing countries such as those in Asia and South America where relapsing malaria is endemic. “The test exists,” says Gonzalo Domingo, a scientific director at PATH, a nonprofit global health organization in Seattle, “but it is quite complicated, and it requires quite complicated laboratory facilities.”
PATH funds companies that are developing a new version of the test for use in rural areas. Field trials of a prototype test that can be performed in two minutes with just a finger prick are under way in Ethiopia, Brazil and India. People with relapsing malaria who test positive for G6PD deficiency instead take a low-dose, eight-week treatment of primaquine that’s less likely to cause side effects.
“It’s a great tool,” Domingo says of the new G6PD deficiency test. “It provides people access to the drug safely.”
Editor’s note: This story was updated August 14, 2018, to include the dosage approved by the FDA, and the clinical trial results related to that dosage.







