Members of the only all-girl, asexual class of animals turn out to be loose. Bdelloidea DNA is tangled up with DNA from all dregs of life – animals, plants, bacteria and even fungi. The shocking finding, to be reported in the May 30 Science, may explain how the group has survived without sex for more than 35 million years.
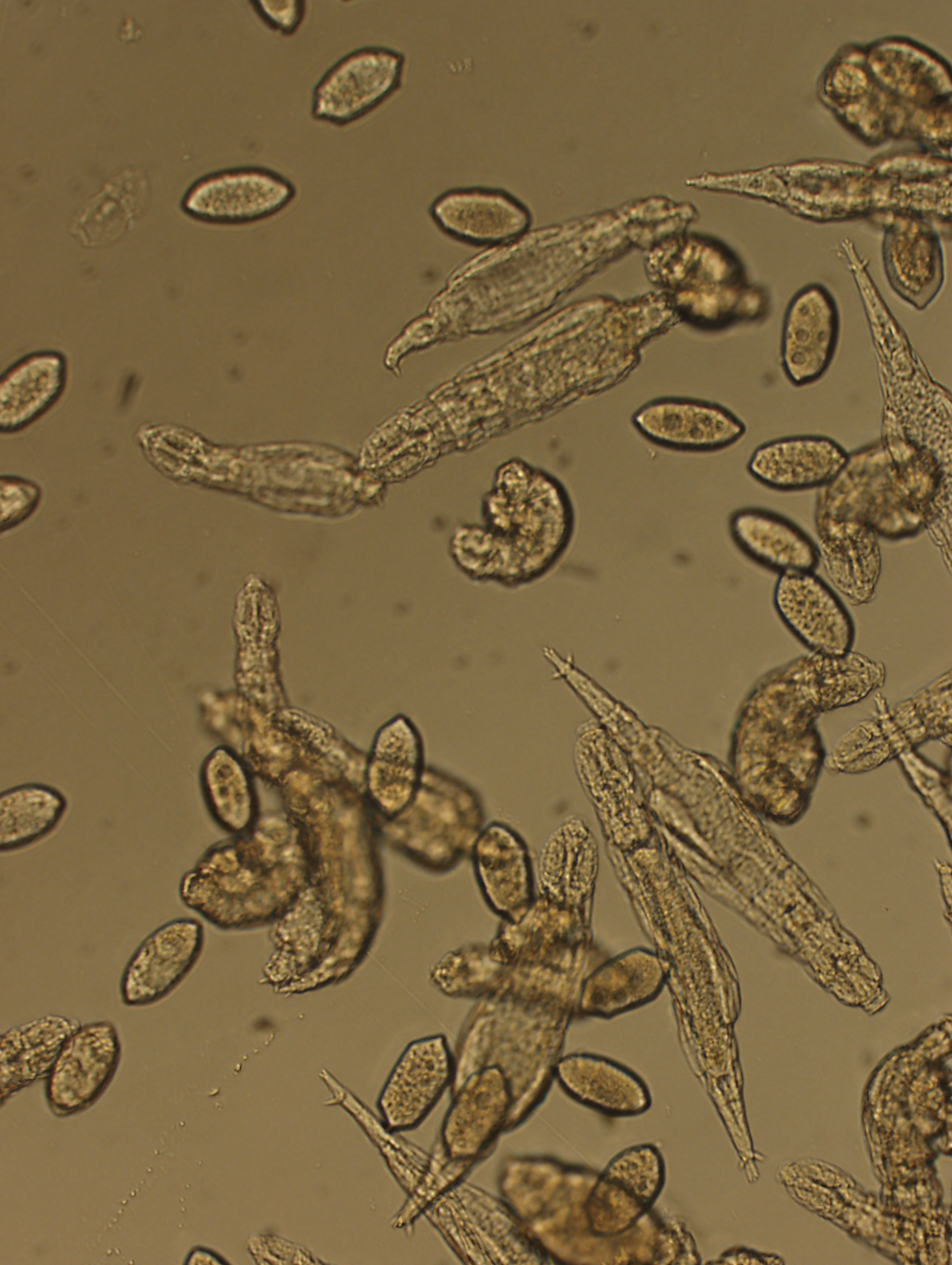

Sex generates genetic variation because genes in mom’s egg and dad’s sperm meet to form novel combinations in an embryo. But bdelloid invertebrates undergo no such process. Like most rotifers, bdelloids swirl around in freshwater ponds or droplets on moss, eating teensy particles. Some animals that occasionally reproduce asexually can also sometimes produce sexually. But the entire class of bdelloids are always spermless and exclusively female. The ovary-bearing females lay tiny eggs out of which clonal babies hatch.
So, the bdelloids don’t create new genetic patterns in any traditional sexual sense. Instead, new genes have been woven into the genome by an unusual process called horizontal gene transfer, in which genes hop organism to organism rather than being passed down, parent to offspring.
While horizontal jumps occur often among bacteria, jumps are nearly unheard of in the animal kingdom, says Irina Arkhipova, a molecular geneticist at HarvardUniversity and at the Marine Biological Laboratory in Woods Hole, Mass.
Yet when Arkhipova and her colleagues analyzed one percent of the total bdelloid genome, they found that more than a third of the genes were derived from other organisms, including those as disparate as filamentous fungi and rare proteobacteria.
“Finding that massive parts of a complex animal are derived from other genes is a big thing,” comments Charles Davis, a Harvard botanist who studies horizontal gene transfer in plants. “That’s pretty fun!”
Bdelloid rotifers may pick up genetic hitchhikers during dry spells when the spinsters shrivel up for months or years at a time. In their dehydrated state, cells rupture and genes may break apart, Arkhipova says. When water returns, the rotifers miraculously piece themselves back together. Other non-rotifer genes found in the vicinity may be incorporated into the bdelloid’s chromosomes as they undergo the spectacular repair, the team hypothesizes.
This genetic mishmash might be the start of a new theory on how rotifers have been so successfully celibate. The genetic shuffling associated with sex can unite advantageous genes, resulting in an organism better prepared to adapt to a changing environment. Scientists think that most lineages of asexual animals eventually die because they are genetically too rigid to adapt.
“The abandonment of sex usually leads to extinction,” says Matthew Meselson, who leads the rotifer lab at Harvard. “But the bdelloids survive.”
Adopting foreign DNA may help maintain genetic diversity in the absence of sex. In support of this idea, a number of the alien genes in the rotifer genome have remained intact. When the researchers inserted a bacterial gene found in the bdelloid rotifer into the bacteria E. coli, the gene still encoded the enzyme it usually makes in bacteria to construct cell walls. The gene probably isn’t producing bacterial structures in bdelloid rotifers, but could be doing something new.
Another possibility is that individual rotifers are picking up DNA from other rotifers too, Meselson says. “That would be like a weird form of sex.”
Foreign genes were only found at the tips, or telomeres, of chromosomes, which don’t code for proteins. Like plastic caps on shoelaces, telomeres protect the important inner parts of genetic strands from destruction. Immigrant bits in the middle of the strand, where protein-coding genes reside, might have interfered with the function of genes necessary for rotifer life. In this case, the animals would not have survived and those genes wouldn’t have been passed on to offspring. On the other hand, the foreign elements at the tips might add to protection provided by telomeres. “It’s possible,” Arkhipova says, “that the addition of extra DNA to the ends of chromosomes could even be beneficial.”