Phil Baran finds simple recipes for complex molecules
Chemist’s philosophy is to closely mimic how molecules are produced in nature

Chemist Phil Baran draws on artistry and creativity to efficiently synthesize molecules that could improve people’s lives.
MacArthur Foundation
Phil Baran, 39
Chemist
Scripps Research Institute
 This is not chemist Phil Baran’s first rodeo: It’s clear that he has done media interviews before. In his Scripps Research Institute office perched above a golf course along the Pacific Ocean in La Jolla, Calif., he is at ease, helpful and patient answering basic questions — why is it important to develop a new way to make a carbon-carbon bond? — as well as opining about how chemists would be better off going after more private research funding, why mentorship is still the best model for science training and his sense that the public (unjustly) thinks of chemistry as a bad word. People take for granted the many products that we rely on every day, he says. At 39, Baran is still young but already experienced and accomplished enough (he has been a tenured professor with his own lab for more than a decade) to be wary of the breathless attention journalists and prizes can bring.
This is not chemist Phil Baran’s first rodeo: It’s clear that he has done media interviews before. In his Scripps Research Institute office perched above a golf course along the Pacific Ocean in La Jolla, Calif., he is at ease, helpful and patient answering basic questions — why is it important to develop a new way to make a carbon-carbon bond? — as well as opining about how chemists would be better off going after more private research funding, why mentorship is still the best model for science training and his sense that the public (unjustly) thinks of chemistry as a bad word. People take for granted the many products that we rely on every day, he says. At 39, Baran is still young but already experienced and accomplished enough (he has been a tenured professor with his own lab for more than a decade) to be wary of the breathless attention journalists and prizes can bring.
That attention has come in many forms, notably a MacArthur Fellowship (worth $625,000) in 2013 and more than a dozen other awards, the most recent one from the New York Academy of Sciences. He’s been featured in write-ups in Forbes, Chemical & Engineering News, Chemistry World magazine and The Scientist, among others. He travels often, consulting with a long list of Big Pharma companies. His lab has a blog, Open Flask, and he has cowritten an e-textbook aimed at research chemists. He is frequently mentioned on organic chemistry insider blogs and appears to have his own devoted internet troll (a sure sign of fame). He has even been featured in a local fashion shoot for Modern Luxury. Reporters often call him for comments on other people’s work. But when I met him in his office this summer, he didn’t dwell on any of this.
What Baran wants to talk about is chemistry. “Phil is extraordinarily passionate about his science,” says Andrew G. Myers, a synthetic chemist at Harvard University. “It is clear that he is driven by his love of chemistry.”
Baran’s specialty is designing sophisticated chemical routes to concoct large, complex molecules from scratch, or from commercially available starter materials. It’s like cooking, but much more precise and finicky. His field, known as total synthesis, largely targets natural molecules first isolated from plants or other organisms. Such molecules are used in everything from fragrances and flavorings to pesticides, and they are hugely important in the drug development pipeline.
Most such molecules are easier and cheaper to extract from their natural sources than to make in the lab. But in some cases the plants might be rare (as was the case with the Pacific yew tree that produces the anticancer drug Taxol) or yield small amounts insufficient even for testing. Synthetic techniques also enable scientists to create closely related derivatives of a natural molecule, which may prove more useful than the original. A few decades ago, total synthesis targeted natural molecules as a tour de force of pure chemical prowess, earning accolades and advancing techniques but concocting pathways too complicated and costly to be practical. Baran’s work represents a shift in the field.
“I’m less interested in proving that something can be made chemically,” Baran says. “We want to invent new chemistry that will be useful.”
The lab is Baran’s element. It’s a calling, he says, what he can’t wait to get back to. “I have always found playing with chemicals an escape of sorts,” he says. He doesn’t recall ever being that interested in science as a child, and was not a motivated student until he found science as a high school sophomore, taking an astronomy class at a local community college in Florida, where he grew up. While simultaneously earning his high school diploma and an associate’s degree, and working long hours at Friendly’s, he took high school chemistry, where his teacher indulged his interest in reactions by letting him drop in to the lab and do experiments. “I liked mixing things and making new forms of matter. It was really cool,” Baran says.
We summarize the week's scientific breakthroughs every Thursday.
As an undergraduate, he studied with chemist David Schuster at New York University, where he would spend long hours in the lab, and chemistry became more important to him than breathing oxygen, he says. “There was no end goal. I was more like an artist.” He did, however, become fluent in the language of molecules. From there, he got a spot in the Ph.D. program at Scripps, where he worked in the lab of synthetic chemist K.C. Nicolaou, a giant in the field. “I was an animal,” Baran says. “I had a singular purpose.” It wasn’t until his third or fourth year of graduate school that he even went to the beach.
Earning his doctorate at 24, he did a postdoc with another star of organic synthesis, E.J. Corey, a Nobel laureate at Harvard. “As a graduate student, you get paid to play in the sandbox, essentially. You are starting to correlate reality with your imagination,” Baran says. “But as a postdoc, you have real responsibility. You have to translate ideas in your head to things in the hood.”
That responsibility jumped again when, at age 25, Baran returned to Scripps to run his own lab. “It’s exhilarating and also terrifying.” Managing and funding a team, and dealing with not just your own lab failures but other people’s, that was new and challenging to someone who had lived for the chemistry itself. Now, the focus is on “sustaining the momentum,” Baran says. “I’m constantly thinking about what’s next.”
He claims he works less now than he once did, but he’s still at the lab 12 hours a day during the week, and half a day on Saturday. Surrounded by drawings made by his three young children, an old-fashioned candy dispenser filled with jelly beans and a sleek black desk, he prints out just a few of his team’s recent papers. Science, Nature, Science. A whiteboard covers the wall across from his desk — it’s littered with drawings of chemical structures, reaction designs as well as cartoons, including one of a Santa Claus the size of a fire hydrant. None of this hides Baran’s intensity. “His productivity is nothing short of remarkable,” Myers says.
Many of his team’s more than 100 papers describe quicker routes to synthesizing a molecule. He and colleagues have cooked up ingenol, a molecule whose derivatives have antitumor potential, in 14 steps and were among the first to synthesize the technically complex palau’amine in 25.
Baran does this by a kind of high-risk, fearless approach to reactions, says his postdoc Joel Smith — instead of designing elaborate chemical routes filled with detours, added to protect one part of a molecule while changing another, for example, he looks for more direct paths. “He’s looking for something creative, what he calls ‘powerful,’” says Smith, who has been working on making thapsigargin, an intimidating-looking molecule made previously in a 42-step synthesis, in a new 11-step protocol.
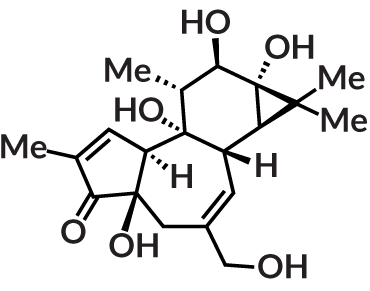
In another example, Baran’s team found a new way to make phorbol, a molecule chemists have known about for more than 80 years. For the last 40 years, they’ve struggled to build it efficiently. Last April in
Nature
, Baran’s group reported a route that would create the complex molecule in
— a huge improvement over the 40 to 52 steps scientists had needed before. Like most products first isolated from plants or other natural sources, phorbol is gangly, a jungle gym of a molecule with a basic structure of carbon dotted with other chemical groups, and (to a synthetic chemist) a disturbing number of oxygens in inconvenient spots. But molecules related to phorbol are of great interest to pharmaceutical companies.
Drawing inspiration from biological systems, Baran’s team relied on a new strategy, based on a two-step technique used to successfully synthesize ingenol in 2013. It involves first building the carbon skeleton (in phorbol’s case, that’s 20 atoms) and then adding the oxygens — mimicking the way that natural enzymes build the molecule within plants. The result still doesn’t compete with natural product isolation in terms of yield, but the strategy will be helpful for creating related molecules.
His philosophy, outlined in 2010 in a well-known Journal of Organic Chemistry paper on what he and coauthor Tanja Gaich call “ideality,” is to hew closely to the way that nature makes molecules. Sometimes this means recombining older techniques in new ways or inventing new chemical strategies. In January in Science, for example, he reported on a new technique to add “spring-loaded” carbon-carbon and carbon-nitrogen bonds to certain kinds of ringed molecules important for drug discovery. In May, also in Science, Baran’s team described a way to use alkyl carboxylic acids, cheap starting materials that are easy to work with, to introduce new carbon-carbon bonds to complex molecules.
Baran says he wants to choose targets for synthesis that have the most potential to help people, which is why so much of his work is now done in collaboration with companies like Pfizer, Bristol-Myers Squibb and the Danish firm LEO Pharma. Like many in his field, he’s drawn to solving real-world problems. But it’s also obvious that Baran finds the creativity of synthetic chemistry the biggest draw. “Total synthesis is a venture into partly unknown territory,” he says. “It’s like building a house while trying to juggle and play chess in 15 dimensions all at the same time.”







