3-D maps of a protein show how it helps organs filter out toxic substances
Changes in pH make the protein open and close in different environments
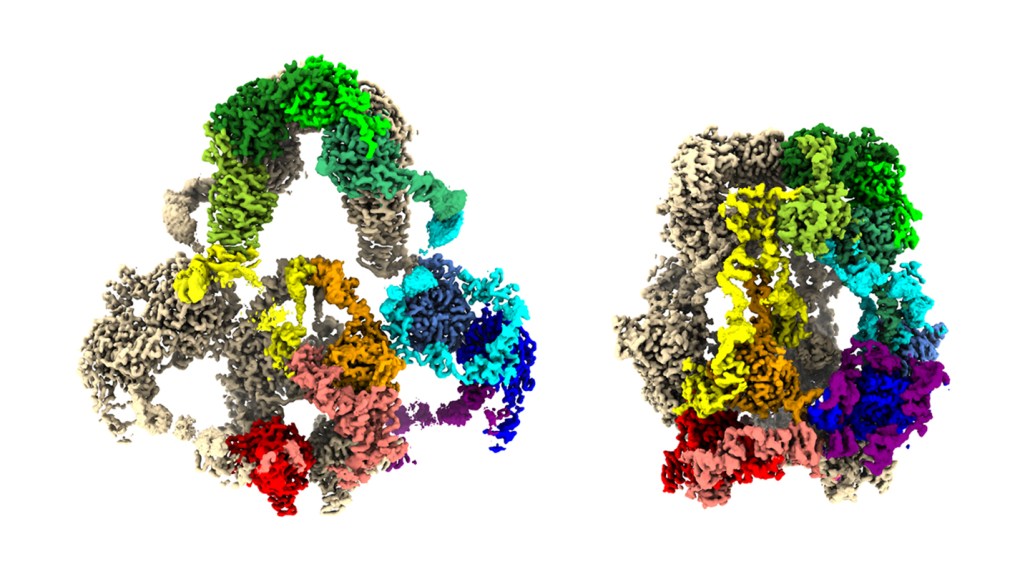
Computer-generated 3-D maps of the LRP2 protein reveal its open shape at near-neutral pH (left) and its closed shape at acidic pH (right). LRP2 is made of two copies of one protein, one shown in tan and the other colored by its different regions.
A. Fitzpatrick, A. Beenken and L. Shapiro/Columbia's Zuckerman Institute







