In Pursuit of the Briefest Beat
Attosecond pulses of light could open electrons’ fast-paced world
In his mind, Paul Corkum envisioned a dramatic thriller. Its actors were the pulsating electric fields of ordinary infrared laser beams and the electrons of atoms in the laser’s path. As the plot unfolded, a puzzle would be resolved — opening, he realized, a new frontier in the measurement of the ultrafast and the ultrabrief.
Corkum is a laser and plasma physicist with Canada’s National Research Council and the University of Ottawa. His vision, in 1993, led him to become a pioneer in a field called attosecond science; since then his work has won him a stack of Canada’s top science prizes. He directs the newly opened Joint Laboratory for Attosecond Science in Ottawa. The laboratory produces X-ray flashes quick enough to “freeze” electrons orbiting an atom, and claims to make Ottawa the attosecond capital of the world.
“I tell my students we are puppet masters,” Corkum says. “We have access now to forces on charged particles that are on the scale of those that hold matter together, and we can make them do what we wish, with exquisite accuracy.”
Someday, attosecond science could lead to a deeper understanding of the chemistry of life, photocells of vastly greater efficiency or even electronic circuits managed by nanoscale lasers. But for now, this nearly two-decade-old science is still doing warm-up calisthenics before its first real game.
Attosecond science’s current lack of major discoveries bears some resemblance to the Large Hadron Collider just going through its shakedown exercises on the French-Swiss border, or the Hubble Space Telescope back when it sat on the launchpad — loaded with delicate mirrors, magnets, electronics, spectroscopes, cameras and the hopes of a corps of scientists already excited despite no achievements yet in hand. There is also a big difference. Attosecond research has no glamorous instrument to capture popular imagination. The key to its allure lies within its name.
Short and shorter
An attosecond is 10-18, a billionth of a billionth, of a second. An attosecond is to a second as a second is to the age of the universe. In three attoseconds, a beam of light traveling 300,000 kilometers per second can get only from one side of a water molecule to the other. And the electron of a hydrogen atom, dissolved in a hazy cloud of quantum mechanics probability, sloshes from one side of the atom to the other every 24 attoseconds — a fundamental oscillation dubbed the atomic unit of time.
The attosecond scale stretches from one to 1,000 attoseconds, at which point the slower femtosecond domain begins. A pulse of one femtosecond, 10-15 (a millionth of a billionth) of a second, is still pretty fast. Ahmed Zewail, a physicist and chemist at Caltech, netted a 1999 Nobel Prize for using femtosecond pulses of laser light to study how molecules reflect and refract light, and otherwise behave at these fast time scales. A primary achievement of femtosecond science was to reveal how quickly the nuclei of atoms in molecules rearrange during a reaction.
“But we knew already that before the nuclei start to move, the electrons’ locations have already changed enormously,” Corkum says. To see that in action would require going faster than a femtosecond, and by the early 1990s technology had not yet caught up with theory enough to do so.
It was a pause in the science of taking snapshots of nature that had begun in the 19th century when cameras first froze horses in mid-gallop, revealing to science and to art that all four feet are regularly in the air simultaneously. Over time, faster and faster strobe lights and then laser flashes permitted images of processes to be captured within a thousandth, a millionth and shorter fractions of a second. But for making signals shorter than the femtosecond range for snapshots of electron activity inside atoms and molecules, even the best laboratory lasers had wavelengths a hundred times too long. The problem was getting enough power to run lasers at ever-decreasing wavelengths; the power required goes up by a factor of 100,000 every time the wavelength goes down by a factor of 10.
By the late 1980s, however, physicists and optical researchers — first a team led by Charles Rhodes, who was then at the University of Illinois at Chicago — had noticed hitchhikers on ordinary red laser light after it went through thin vapors of gaseous atoms. Embedded were weak but unmistakable extra signals with really short wavelengths and frequencies thousands of times higher — in the far ultraviolet, bordering on X-rays. No one could explain the mystery process and it got a generic name: high harmonic generation.
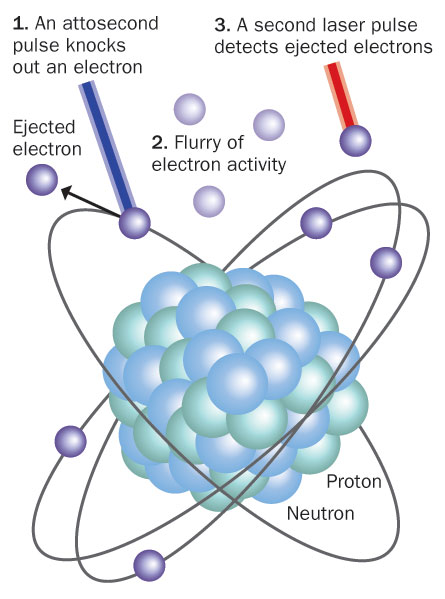
It was a puzzle, and Corkum cut the knot. As he explains now, he imagined the laser beam’s uniformly pulsating electric field as “reaching out its hand” as it encountered certain simple atoms. The hand grips an electron vulnerable to the frequency and quantum energy of the laser light’s photons. The hand then pulls the electron nearly instantaneously away; it reaches a velocity of thousands of kilometers per second in a twinkling. The electron’s precise locale is inherently imprecise, in accord with the particle-wave duality of the atomic world, but not so hazy that the laser’s electric field can’t take hold of its negatively charged locale and sweep it hundreds of times the atom’s radius away.
The action is similar to the way light knocks electrons entirely out of metals in the photoelectric effect that Albert Einstein explained in 1905, later earning a Nobel Prize. But in his insight, Corkum saw a twist in that classic plot. On the brink of permanently liberating its kidnapped cargo and leaving behind an ionized atom short one electron, the laser’s throbbing electric field cleanly reverses direction. It must do so — after all, a laser beam is composed of well-ordered electromagnetic radiation, its electric and magnetic components vibrating back and forth in unison. Corkum’s imagined hand violently punches the electron right back into the atom whence it came, again at near light speed. The atom shudders as the process, formally called recombination, plays out.
Most important, the atom sheds the incoming electron’s extra energy. It does so via a brief, intense burst of exceedingly short far-ultraviolet radiation, or even X-rays. What’s more, as the laser beam washes through the gas in its path, the same thing happens — in step — to millions, even billions, of other identical atoms. Their high-frequency electromagnetic distress chirps merge into a rising chorus in perfect tune. The accumulating signal tucks itself into the longer-wave laser beam as it continues on its path. This electromagnetic melody, in its spectrum and structure, carries with it detailed information on what just happened.
By the nature of the process, it does not make a continuous signal, but one prepackaged into exceedingly short pulses of high frequency — the hitch-hikers that had been seen on the red laser light. “Within days, I understood this was a route to attosecond physics,” says Corkum. “This changed my career,” he adds — as it changed careers for a cadre of other physicists and theoretical chemists around the world.
Fast physics
Notable among those who plunged into the field was Hungarian-Austrian experimental physicist Ferenc Krausz and his group at the Max Planck Institute of Quantum Optics in Garching, Germany. Among Krausz’s key roles has been to develop special multilayer “chirped” mirrors. Their surfaces put delay times into the overlapping waves in short pulses of infrared light, piling the waves into even shorter blinks.
Usually, pulses of light act like luminous sausages racing through optical equipment end-first. But femtosecond pulses, and especially attosecond pulses, are more like pancakes flying face-first. Their thickness is measured in billionths of a meter, but their width is a thousand times or more their thickness.
Rapid improvement also has come in the ability to control the individual waves in the femtosecond laser pulses — leaving in each a single high peak, like a solitary rogue wave that rises at sea and threatens ships. “We have been able to realize pulses for the first time that consist of only one or two wave cycles,” Krausz says. “This was the key to making an isolated attosecond pulse.” In his office Krausz has a certificate given to his team in 2008 for generating “the shortest ever flash of light,” a pulse 80 attoseconds long. For now, the record stands.
Last year in Reviews of Modern Physics, Krausz wrote that while many expect that photons will become the most powerful tools for computing, “the advance of science and technology by research on electrons is by no means over. On the contrary, it is just beginning.” He expects the field soon to be able to steer electrons inside molecules wherever a researcher might desire, sampling conditions or changing them.
“With disease, it always has its origin in some electronic motion; somewhere in the DNA or an organelle, something breaks. If we want to understand illness at the most fundamental level, we must be able to look at electronic motions,” Krausz says. “Another example: You could ask yourself what are the limitations of electronics. Today electrons switch billions of times per second in a computer. So my personal belief is that the ultimate limits will be close to light frequencies.” And that, he says, would mean a leap in switching speeds by a factor of a thousand.
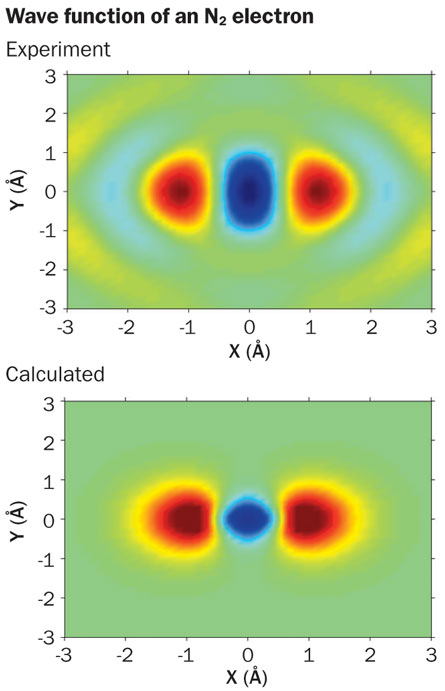
Already some new science has emerged. A team from the University of Colorado at Boulder, led by the wife-husband team of Margaret Murnane and Henry Kapteyn, used laser light to smack molecules of two nitrogen atoms joined to four oxygen atoms, causing vibration. By probing the atoms with combinations of attosecond and femtosecond pulses, the scientists were able to deduce how the electrons sloshed among energy levels with each cycle of vibration that stretched and then squashed the molecules. Other work has led to the first snapshot of the electron orbitals of a two-atom nitrogen molecule, N2.
The assemblages that do such things are not, strictly speaking, as elegant to look at as, say, a giant accelerator or a telescope. An attosecond layout on a lab bench is more of a labyrinth, but one of extraordinarily precise passages. It starts simply enough, with a specialized laser in a metal box about a foot wide and two feet long. Next are rank after rank of mirrors, narrow tubes, wiggling electric field gauntlets, steel chambers and glass light-beam conduits no thicker than angel-hair pasta. Here and there are pumps working hard to keep vital stretches in near-vacuum conditions at the same time that puffs of gas are emitted. “Physics is a collection of good tricks and bad jokes,” says Phil Bucksbaum, a Stanford University physicist. “And we play a lot of tricks on nature in this field.”
This isn’t Big Physics, but it’s not cheap either. The one-room laboratory of theoretical chemist Stephen Leone in the Lawrence Berkeley National Laboratory cost perhaps $5 million, including roughly $750,000 for the laser. “Each of those chirped mirror compressors is maybe $10,000,” said Phillip Nagel, a University of California, Berkeley graduate student working in Leone’s lab, as he pointed at an array that would look fine in a pinball machine.
Leone and his students have been trying for several years to put a stopwatch on a molecule of sulfur hexafluoride as it falls apart after being smacked by an attosecond-class pulse.
“This will be new science, and it will be a good demonstration,” Leone says. “The thing about this molecule is that if you ionize it — just knock one electron out — it totally upsets the whole bond structure.”
Electron snapshots
A close look at this one test provides a taste of how the whole field works. The molecule starts out as a sulfur atom nestled among six of fluorine. But how much time elapses before SF6 becomes the ion SF6+ and then becomes drifting pieces of SF4 or smaller pieces? And, more important, after the moment of ionization, when and in what order do remaining electrons begin unlashing the bonds that held the molecule together? If such things can be learned in this simple system, it would help lead to the creation of new, complex chemical systems and products that take full advantage of the capacity of atoms to rearrange and recombine.
To find out, Leone and his group tune their laser and its downstream system of chirped mirrors and other gadgets to produce infrared pulses about seven femtoseconds (7,000 attoseconds) long. The pulses first pass through a cloud of neon gas — about a billion atoms’ worth — in a sturdy steel vacuum chamber. As they do so, the neon atoms each suffer the sort of fate that Corkum first imagined nearly two decades ago.
Electrons in vulnerable orbitals get yanked far from the atoms’ nuclei and then smashed back in. Each rattled neon atom spits its brief package of ultraviolet rays as the electrons recombine. The result is that the onrushing laser beam’s pulses continue on their way, each carrying within it a far-ultraviolet pulse about 450 attoseconds in duration.
Filters then separate and isolate the attosecond pulses. These are given the job of being pump pulses. Each is directed down another conduit to a second, near-vacuum reaction chamber a few feet away. There, each blasts through a waiting wisp of SF6 molecules, ionizing them. The pulses are so short that Leone and his team know exactly when the ionization occurs. Thus they have a time zero for their atomic stopwatch.
The final step is to figure out what the electrons are doing as the molecules disintegrate. That’s the job of probe pulses — additional, seven-femtosecond pulses of infrared laser light. They ionize the mortally wounded molecules and their remnants. They do it again and again, with different time gaps between the pump and probe pulses.
Analysis of the spectra and other qualities of these rounds of evicted electrons should permit the team to, in effect, make a slow-motion movie of the fast-changing electronic bond structures. “Simple, huh?” Nagel said, as he looked at the setup.
It is still a work in progress. “The electrons don’t seem to be entering our detector correctly,” he mumbled.
As the field gradually moves from launchpad to practical, routine use, many have their eyes on the next stages. First will be development of immense centralized facilities for certain kinds of attosecond research.
Some large research institutes expect to turn to ultrapowerful linear accelerators hundreds of meters to kilometers long to create a type of laser called a free-electron X-ray laser. Such lasers are millions of times more powerful than the current tabletop infrared lasers that are milking details from little clouds of atoms. The SLAC National Accelerator Laboratory in Menlo Park, Calif., last year demonstrated the first such system, and Europe plans to open its XFEL laser facility near Hamburg, Germany, in 2014. (But such facilities are costly and uncertain. The United Kingdom just canceled its big free-electron laser, the New Light Source.)
Not far from Leone’s lab another Berkeley team is laying plans for a possible “Next Generation Light Source.” Under the hills would be an underground linear accelerator more than half a mile long and with 10 or so free-electron lasers for rent to visiting researchers. Attosecond pulses, says the lab’s officer in charge, physicist Roger Falcone, will be routine parts of the operation. The cost: $1 billion–plus from the federal government. At the soonest, the light source could be ready in 10 years.
In the meantime, small-scale attosecond science will plunge forward. Its researchers are thinking of the next frontier in the science of brevity: zeptosecond pulses. A thousand times shorter, they might track not just motions of electrons, but of quarks inside the nuclei of atoms. Asked how to do that with his accelerator, Falcone says, “I have no idea.”
“I have an idea,” Corkum responds to the same question. “We’d start with an electron in a long-wave laser, just like now. Only we’d pull it much farther from the atom, and then drive it all the way into the nucleus.” He hasn’t worked out the rest in detail. But someday somebody surely will try. Stay tuned.