Spinal stimulation gives some people with paralysis more freedom
Electrodes help reroute nerve signals around trouble spots

As part of a small clinical trial, Michel Roccati uses an implanted electrical stimulator to activate his spine, allowing him to move around without a wheelchair after his spinal cord injury.
Jimmy Ravier/EPFL
By his count, Michel Roccati is on his third life, at least. In the first, he was a fit young man riding his motorcycle around Italy. A 2017 crash in the hills near Turin turned him into the second man, one with a severe spinal cord injury that left him paralyzed from the waist down. Today, the third Michel Roccati works out in his home gym in Turin, gets around with a walker and climbs stairs to visit a friend in a second-story apartment. Today, he says, his life is “completely different than it was before.”
Roccati, age 31, is one of three men who received experimental spinal cord stimulators as part of a clinical trial. All three had completely paralyzed lower bodies. The results have been a stunning success, just as Roccati had hoped. “I fixed in my mind how I was at the end of the project,” he says. “I saw myself in a standing position and walking. At the end, it was exactly what I expected.”
The technology that Roccati and others use, described in the February Nature Medicine, is an implanted array of electrodes that sits next to the spinal cord below the spot severed by the injury. Electrical signals from the device replace the missing signals from the brain, prompting muscles to move in ways that allow stepping, climbing stairs and even throwing down squats in the gym.
Today, Roccati spends time working at the consulting company he owns with his brother and sharing his ongoing physical accomplishments with researchers. “Every week we get a WhatsApp from Michel doing something new,” says study coauthor Robin Demesmaeker, a neural engineer at NeuroRestore, a research and treatment center in Lausanne, Switzerland.

These results and others prove that, with the right technology, people with severe spinal cord injury may be able to stand up and walk again. It’s a remarkable development.
But the really big news in this area goes far beyond walking. Many people with spinal cord injuries deal with problems that aren’t as obvious as paralysis. Low blood pressure, sexual dysfunction and trouble breathing or controlling hands, arms, bladder and bowels can all be huge challenges for people with paralysis as they navigate their daily lives. “These are the things that actually matter to people with spinal cord injuries,” says John Chernesky, who has a spinal cord injury. He works at the nonprofit Praxis Spinal Cord Institute in Vancouver, where he makes sure the priorities and voices of people living with spinal cord injuries are heard and addressed in research.
By figuring out the language of the spinal cord, researchers hope to learn how to precisely fill in the missing commands, bridging the gap left by the injury. The work may pave the way to treat many of these problems flagged by patients as important.
“The research field is changing … embracing all these other aspects,” says neuroscientist Kim Anderson Erisman of MetroHealth Medical Center and Case Western Reserve University in Cleveland. Already, early clinical trials are tackling the less obvious troubles that come with spinal cord injuries. Some of the same scientists that helped Roccati recently showed that similar spinal cord stimulation eased a man’s chronic low blood pressure. Other researchers are improving bladder and bowel function with stimulation. Still more work is focused on hand movements. The technology, and the understanding of how to use it to influence the nerves in the spinal cord, is moving quickly.
Not coincidentally, the way the research is being conducted is shifting, too, says Anderson Erisman, who has a spinal cord injury. “Scientists know the textbook things about spinal cord injuries,” she says. “But that’s not the same thing as living one day in the life with a spinal cord injury.” Involving people with such injuries in studies — as true partners and collaborators, not just subjects — is pushing research further and faster. Such collaboration, she says, “will only make your program stronger.”
These efforts are in the early stages. The stimulators are not available to the vast majority of people who might benefit from them. Only a handful of people have participated in these intense clinical trials so far. It’s unclear how well the results will hold up in larger trials with a greater diversity of volunteers. Also unclear is how attainable the technology will be for people who need it. For now, the research often requires large teams of experts, typically in big cities, with patients needing surgery and months of training the body to respond.
Still, the promise of spinal cord stimulation extends beyond spinal cord injuries. Stimulating nerves on the spinal cord could help people with symptoms from strokes, Parkinson’s disease, multiple sclerosis, cerebral palsy and other disorders in which signals between the brain and body get garbled. Initially, “hardly anyone wanted to believe these [improvements] were happening,” says V. Reggie Edgerton, an integrative biologist at the University of Southern California’s Neurorestoration Center and the Rancho Los Amigos Rehabilitation Center in Downey, Calif. “But now, they’re happening so regularly that it’s undeniable.”
A turnaround
Not so long ago, a serious spinal cord injury was a death sentence. “Prior to World War II, the life expectancy of a person with a spinal cord injury was measured in days or weeks,” Chernesky says. If the injury didn’t kill a person directly, they’d often succumb to respiratory distress or blood poisoning from a bladder infection. “If you lived six months, that was impressive,” he says.
The spinal cord ferries signals between brain and body. Signals from the brain tell leg muscles to contract for a step, blood vessels to expand and the bladder to hold steady until a bathroom is within reach. Signals from the body to the brain carry sensations of moving, pain and touch. When the spinal cord is injured, as it is for an estimated 18,000 or so people each year in the United States alone, these signals are blocked.
Lost signals
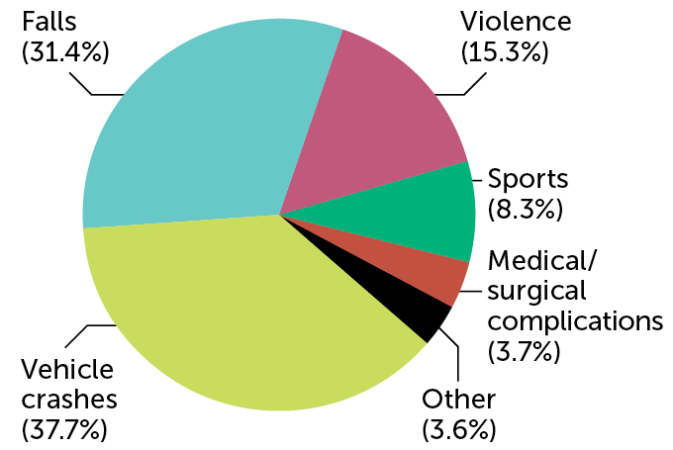
In the United States, an estimated 18,000 people suffer a spinal cord injury each year. Vehicle crashes and falls are the most common causes, data collected from 2015 to 2021 show. Violence, particularly gunshot wounds, and sports accidents are also common reasons.
Causes of spinal cord injuries in the United States
Researchers have long dreamed of repairing the damage by bridging the gap, perhaps with stem cells or growth factors that can beckon nerve cells to grow across the scar. The idea of using electricity to stimulate nerves below the site of the injury came, in part, from an accidental observation. In the mid-1970s, scientists were testing spinal cord stimulation as a treatment for severe and chronic pain. One participant happened to be a woman who was paralyzed from multiple sclerosis, a disease in which the body attacks its own nerves. With the device implanted on her spinal cord to ease pain, she was able to move again. That surprising discovery helped spark interest in spinal cord stimulation as a way to restore movement.
In 2011, researchers at the University of Louisville in Kentucky restored the ability to stand to a 23-year-old man with paraplegia. In 2018, that group and two others reported even greater strides in spinal stimulation: People with severe spinal cord injuries could step and walk with assistance (SN: 12/22/18 & 1/5/19, p. 30).
Earlier this year, Demesmaeker and his colleagues, including Grégoire Courtine of the Swiss Federal Institute of Technology in Lausanne, published the achievements of Roccati and two other men. All three men had been unable to move their lower limbs or feel any sensations there.
Most previous studies had relied on an electrode array designed and approved by the U.S. Food and Drug Administration to treat chronic pain. That device has electrodes that are implanted along the spinal cord, where their electrical jolts can ease long-term pain in the back and legs. But Roccati and the two other men received a specially designed device that was slightly longer and wider than that earlier device, able to cover more of the spinal cord’s nerve roots and provide more stimulation options.
Several weeks after surgery, the men visited the laboratory in Lausanne to start searching for the optimal stimulation settings. The timing, pattern and strength of the electrode signals were adjusted to allow Roccati to move. “We found a good sequence with the engineers that allowed me to stand up and see my body standing in the mirror in front of me,” Roccati says. “It was a very emotional moment. A standing ovation appeared from everyone in there.”
That first day, he took steps with the stimulation while being supported by a harness. That quick improvement is important, says biomedical engineer Ismael Seáñez of Washington University in St. Louis. “From day one, you can start training.” After months of intense practice (four to five sessions a week for one to three hours at a time), Roccati could walk without the harness, using only a walker.
The men in the trial have all been getting stronger, even when the stimulation is off. That suggests that there’s some sort of repair happening in the body, perhaps due to stronger neural pathways in the spinal cord. Just how the stimulation repairs the spinal cord is one of the big remaining mysteries.
“It’s exciting to see,” Seáñez says. “But it’s a first step in all of the different challenges faced by people with spinal cord injuries.”
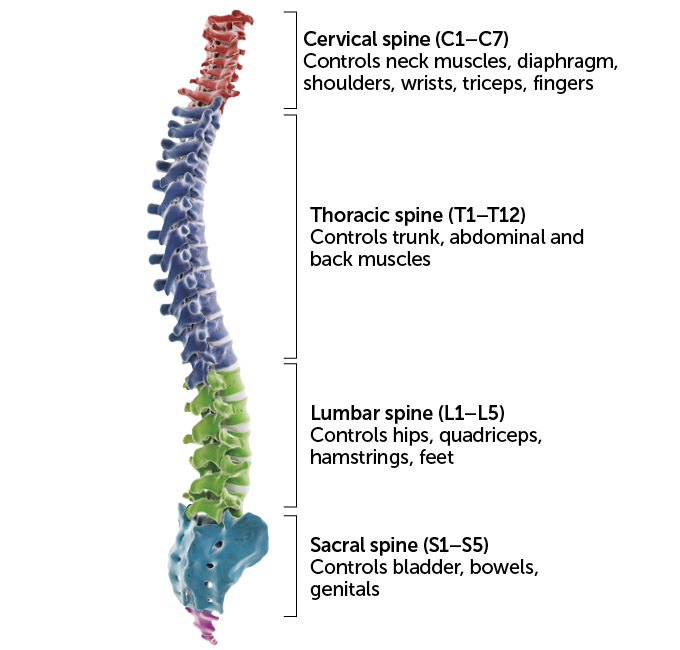
Spinal control
Nerves in each spinal cord region carry signals to and from different body parts. That means the outcome of an injury depends on its location, with lower injuries affecting less of the body.
Spinal cord control over parts of the body

Signaling blood vessels
One important problem with paralysis is low blood pressure. When the spinal cord is damaged, the signals that keep blood vessels constricted and blood pressure normal can get lost. Low blood pressure can leave people mentally foggy, exhausted and prone to fainting, not ideal conditions for physical rehab work. Blood pressure can also rise or fall quickly, upping the risk for stroke and heart attack. That’s a huge problem, says Aaron Phillips, who studies the physiology of the nervous system at the University of Calgary in Canada. “Blood pressure is one of the vital signs of life,” he says.
So Phillips, Courtine and colleagues decided to implant a spinal cord stimulator to see if it would help a man who had low blood pressure due to a spinal cord injury. When the machine was on, his blood pressure rose toward normal levels, the researchers reported last year in Nature. When the stimulation was turned off, the man’s blood pressure dropped.
The scientists homed in on an area in the mid-back, just around thoracic segment 11 in the human spine. That spot had the biggest effect on the man’s blood pressure. “We now know that there’s a key area in the spinal cord that, when stimulated, controls neural circuits and the connected blood vessels to elevate and decrease blood pressure,” Phillips says.
The system the researchers developed operated like a thermostat with a set point. In experiments with the man on a tilting table, monitors sensed low blood pressure when the table mimicked standing up. That triggered the stimulators, which in turn told the blood vessels to bring the pressure back up to an acceptable level.
The results represent “a huge pinnacle of my career,” Phillips says. But many challenges remain. The system used in the study in Nature needs tweaking, and the long-term effects of such stimulation aren’t known. Phillips and his colleagues hope to answer these questions. With funding from DARPA, a U.S. Department of Defense agency that invests in breakthrough technologies, the team is working on a wireless blood pressure monitor, and an upcoming clinical trial aims to enroll about 20 people with spinal cord injuries that affect their blood pressure.
Patient priorities
In 2004, Anderson Erisman and her colleagues asked people with spinal cord injuries to share their priorities for regaining function. For people with quadriplegia, who have impairments from the neck down, hand and arm function were most important. For people with paraplegia, who have use of their arms and upper body, sexual function was the highest priority. Both groups emphasized the desire for restored bladder and bowel function, Anderson Erisman and colleagues reported in the Journal of Neurotrauma. Walking was not at the top of either group’s wish list.
That’s no surprise to Chernesky, who uses a wheelchair. “The general population looks at people with spinal cord injuries rolling around in wheelchairs, and they say, ‘Oh, poor bugger. I bet he wishes he could walk,’ ” he says. “They have no idea that quite rapidly after an injury, walking becomes a lower priority.”
Chernesky himself recently participated in a clinical trial designed to externally stimulate the cervical spine, in his neck, to improve arm and hand movements. The device he tested sent signals to the spinal cord through the skin — a less invasive approach than surgery, but one that may sacrifice some specificity compared with implanted versions. Throughout that process, Chernesky noticed improvements in energy, sleep, strength, core stability and movement of both upper and lower limbs.
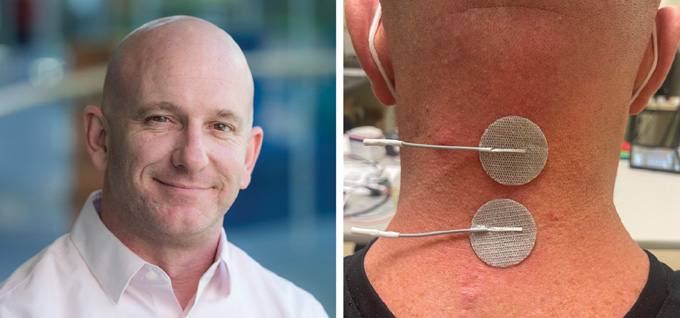
Other scientists are working on similar ways to externally stimulate the spinal cord to improve people’s autonomic nervous system. That system keeps your blood pressure steady, makes you sweat when it’s hot and tells you when you need to head to a bathroom.
In studies at the University of Southern California and elsewhere, Edgerton and colleagues have recently shown that external stimulation improved bowel function. He and others have also seen stimulators improve bladder function in people with spinal cord injuries and strokes. “We know some subjects can now feel when their bladder is full,” says Edgerton, who started a company called SpineX in 2019 to develop the technology further. That newfound sensation gives people enough time to get to the bathroom. “This doesn’t happen overnight, and it doesn’t happen in every individual,” he cautions. “But it happens a lot.”
Getting past the hype
The next phase of research will be boring — in the best possible way. Large, standardized studies will need to address some mundane but crucial questions, such as who might benefit from stimulation, how much improvement can be made for certain symptoms and whether the therapy causes any extra trouble for some people. “This type of technology will go from a very exciting proof of concept to standard clinical care,” Seáñez predicts.
Over his nearly 30 years of living with a spinal cord injury, Chernesky has witnessed enough so-called scientific breakthroughs to be skeptical. He’s immune to hype. But he admits that he’s excited by this moment. “Because now we can reverse paralysis,” he says. That doesn’t mean people are going to suddenly be tap dancing like Fred Astaire or playing a Chopin concerto anytime soon, he’s quick to add. “But every little bit matters.”
Roccati, for one, no longer has to recruit friends to carry him in his wheelchair up stairs to socialize. He feels more energetic. He is working on his summer six-pack abs. He has transformed, again, into someone new. “Now, after the implant, I am another type of person,” he says, a more optimistic version of himself.
This technology is still a long way from helping everyone who might benefit. Still, these stimulators hold great promise. “I am quite hopeful, almost certain, that these devices are going to become available, and there will be a lot of people buying them,” Chernesky says. “When you have nothing, and you can get a little bit back — how good is that?”