Telltale Heart
Researchers are uncovering the genetic plan for building a heart
When Eldad Tzahor cut a small piece of tissue from the front end of a chicken embryo and placed it on a glass laboratory dish, he intended to observe, as he had before, how the head develops. He would watch the scrap of tissue grow and change from a mass of anonymous cells into well-defined facial muscles. But what he saw over the next 2 days, as the cells dutifully began to divide and specialize, was a complete surprise.
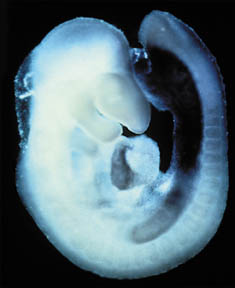
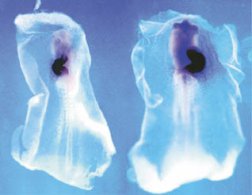
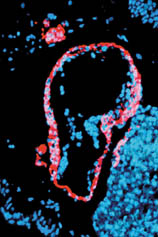
Instead of facial muscles, a tiny heart took shape on his dish. Then, the heart began to beat.
Tzahor’s experiment may have taken a science fictional turn, but there was a simple explanation. Tzahor had accidentally cut out just a little less tissue than he had in his previous experiments. In the absence of a signal from an adjacent region, the cells had followed a different set of instructions and become a heart instead of a face.
Tzahor, who studies biological chemistry and molecular pharmacology at Harvard Medical School in Boston, immediately changed his research direction. He decided to find out which genes control the fate of these cells.
In so doing, he joined a cadre of developmental biologists that focuses on decoding the first steps in an animal’s genetic instruction sheet to make a heart. The accumulation of data is beginning to pinpoint the connections between particular genes and heart problems that are apparent at birth or turn up much later in life.
As these investigators uncover more details about early heart development, they are also answering one of biology’s most fundamental questions: How does a single cell become the integrated community of differentiated cells and tissues that make up a fully formed animal?
In the early stages, cardiac formation is at the heart of the matter.
“The heart is the first organ to develop in a vertebrate embryo,” says Eric Olson, who is a developmental biologist at the University of Texas Southwestern Medical Center in Dallas. “Forming the heart requires an extremely complex series of [shape-changing] events, and small perturbations can have catastrophic consequences.”
Embryologists first became familiar with the major structural changes of heart development in the 19th century, when they observed the growth of frog and chick embryos. Now, however, developmental biologists are revealing the genetic and molecular machinery underlying the differentiation of embryonic cells into specific organs.
“Until 5 years ago, we didn’t know anything about the genes for heart development,” says Olson. Biologists have gradually increased the numbers of new molecular techniques and reference tools at their disposal that help track down these genes.
Using flies, frogs, mice, and zebra fish as models, genetic researchers have unmasked genes and their products for many stages in the molecular cascade of events leading to the finished heart.
“We have reached a critical mass of genes over the last 5 years . . . to identify the key pathways that regulate heart formation,” notes Deepak Srivastavaa , pediatric cardiologist and developmental researcher also at UT Southwestern Medical Center (see In search of answers, below). About 50 genes involved in heart formation have been uncovered, he says.
Making a heart
Three teams, including Tzahor’s, recently described work that pieces together the genetics and molecular signals underlying one of the first steps in making a heart. Their studies indicate that in the early embryo, cells from a tissue called the mesoderm face a clear decision. Should they take the road to becoming heart cells or become facial muscle or blood cells instead?
The three new studies have identified powerful signaling molecules that help direct where and when the heart will begin to form.
The groups studied genes active in the very young embryo that produce members of a family of protein molecules called Wnts (“wints”). Wnt combines the names of two related genes: wingless, which when mutated eliminates wings in flies, and Int-1, a gene that when mutated can cause cancer in people. The Wnt signaling molecules are present at other times in development, but early on, some of them carry the message, “Don’t make heart,” says Tzahor.
The Wnt signal penetrates a swath around the tissue secreting it and prevents tissue with heart-forming potential from developing into a heart.
In the experiment that surprised Tzahor by producing a heart in a laboratory dish, he had omitted tissue from the nearby neural tube, which later forms the spinal cord and nervous system. By secreting Wnts, the neural tube cells sent the section of adjacent tissue that he was studying down the path toward head–not heart–development.
In their recent work, Tzahor and Andrew B. Lassar, head of the Harvard laboratory, identified two genetically encoded signals, both Wnts, from neural tube tissue. The biologists then found that forcing another tissue to produce Wnt molecules could recreate the normal events in the early embryo and suppress heart formation. They reported their findings in the February Genes and Development.
The two other teams of researchers identified several molecules that counter Wnts’ action. They give the command, “Make heart.” Where these molecules overlap with Wnts, the cells face the decision of whether to become heart or some other tissue, says Olson, who wrote a commentary on their papers in the March 23 Science. The outcome depends on the relative concentrations of the two types of signal molecules.
Lassar explains that this is like stepping on a car’s accelerator and brakes at the same time. Where the concentration of Wnt molecules, the brake, is slightly lower than that of molecules carrying the opposite signal, cells begin turning into heart tissue.
Hearts normally form in mesoderm tissue at the front of an embryo. The Harvard Medical School team of Valerie Schneider and Mark Mercola found that they could force mesoderm tissue from the back of frog embryos to make a heart by overwhelming the Wnt signal with either of two other signal molecules. The first is called Crescent because it appears in an embryo in a crescent pattern where the heart will develop. The second molecule, Dkk-1, induces head formation in embryos and was named after the German words for thick head.
In the third study, Martha Marvin, Lassar, and their colleagues from Harvard Medical School and the Whitehead Institute for Biomedical Research and the Massachusetts Institute of Technology, both in Cambridge, similarly created hearts in chick tissue that would normally form blood cells. They used a molecule called BMP, bone morphogenic protein, which also plays a part in bone formation later in development.
Identifying these molecules and the heart-forming zones created where they overlap suggests future strategies for therapies to treat damaged or ailing hearts, Olson suggests. To bolster the failing organ, for example, physicians might manipulate cells not yet committed to an identity into becoming heart cells.
Complex construction
The fully formed heart’s compact appearance belies the complexity of its construction. The newly identified genes are just the first involved in a long process. Carefully scripted genetic events for heart development underlie equally complicated structural stages as the heart grows, branches, and changes shape.
The wonder of watching this pumping machine build itself has inspired many embryologists to enter the field. “Seeing a heart develop and beat for the first time . . . it’s like a religious experience,” Olson recounts. “You are looking at this amorphous, very unformed embryo. Then, early on, a group of cells starts to beat and form a tube. The tube loops and twists into shape. It’s like watching a flower form.”
Understanding the initial step that tells tissue to become a heart may seem far removed from these later processes. In many cases, however, the genes that activate heart development play a later role, as well. When defective, they can create many congenital problems, says Olson. A mutated gene may, for example, produce just enough of its signaling molecule to jump-start heart formation but not enough to prevent flaws later in the process that cause heart defects.
A problem for researchers comes in connecting early and late development. If the genes don’t work at all, no heart develops, or at most, an amorphous ball of cells forms. It would be difficult to connect this type of developmental breakdown with a specific birth defect.
“In human terms, [such an] embryo would probably fail a few weeks into gestation,” says Mercola. The genes that direct heart development early lay out the overall patterning of the embryo. Without the molecular signals differentiating between head and heart, or without a heart to pump oxygen to all regions of the body, the embryo never makes it past being a layer a few cells thick.
“Probably what we see clinically [as congenital heart defects] represents just small fraction of what could go wrong with the heart. Most would end in early miscarriages,” says Srivastava (see In search of answers, below). One-third of all human pregnancies fail early on, often before a woman knows that she’s pregnant, he notes. Of these, about a quarter may have to do with a heart problem, Srivastava says.
Identifying the genetic problems behind these defects may allow physicians to intervene and prevent them, he says. If a genetic problem limits the uptake of some nutrient by the embryo, for example, physicians might prevent it by supplementing the mother’s diet.
In the lab, Srivastava and his colleagues study genetic signals that regulate the later formation of the heart’s chambers and vessels. One of the most common of the birth defects governed by these signals is DiGeorge syndrome, which affects 1 in 4,000 newborns. In the last few years, Srivastava’s lab and others have identified a few genetic suspects for the syndrome (SN: 3/10/01, p. 151). DiGeorge syndrome represents an array of problems commonly including holes in the heart, vessel-branching defects, and facial abnormalities, such as down-slanting eyes and a cleft palate.
Other research has implicated other genes in congenital heart defects. These include Jagged-1, which causes a narrowed connection from a person’s heart to the lungs, and Smad6, which gives mice heart valves made of thickened, jelly-like muscle. Similar valve defects are common, fatal problems in human fetuses.
Early development
Findings on early development may offer new opportunities to treat adult heart disease, as well. Heart cells don’t readily repair themselves. Once damaged, the muscle rapidly scars, impeding its function as a pump.
When the adult heart experiences stress, it reactivates the program of gene expression active in the fetal heart. It’s “as if it’s trying to regenerate,” says Olson.
Piero Anversa at New York Medical College in New York City and his colleagues recently documented human heart cells dividing and moving into damaged tissue after heart attacks. This process wasn’t sufficient to save the lives of the people, however, the researchers report in the June 7 New England Journal of Medicine.
To provide the raw material for heart repair, medical researchers are also introducing stem cells collected from other tissues. These are cells, found in both embryos and adults, that are unspecialized and have the potential to grow into different types of cells (SN: 1/13/01, p. 300). In recent research in mice, stem cells collected from various tissues have migrated to damaged areas and transformed themselves into the appropriate tissue.
If scientists could identify the genes responsible for creating heart tissue in the embryo, they might be able use their signals to direct stem cells to replace damaged cardiac cells, says Mercola.
This approach is controversial. “There is no question that understanding the normal development of the heart will give us very important information about the congenital [heart defects],” says Anversa. But he questions whether conclusions from embryology will be useful for devising treatments for adult disease.
Anversa is also uncertain whether researchers must learn the early signals that direct heart development. He and his colleagues recently repaired the hearts of adult female mice by introducing stem cells from bone marrow of adult male mice. The cells migrated to damaged heart tissue and turned themselves into working cardiac-muscle cells.
The stem cells didn’t seem to need any priming from the researchers to find their way to tissue in trouble or respond to instructions from surrounding tissue telling them to specialize. How they do it, Anversa says, is a mystery.
Some scientists have suggested that studying stem cells native to the heart might help resolve these questions.
They may also provide researchers with information about how genes trigger the original conversion of human embryonic cells into heart tissue, Anversa says.
In the end, it’s the marriage of stem cell research and developmental research that is likely to yield the biggest payoffs in the study of heart development and disease, says Mercola. By figuring out the genes and molecular signals that induce formation of the heart, scientists are likely to reveal the events that induce undifferentiated cells to become healthy heart tissue.
“Learning how the cells accomplish this, one would be able to imitate the process and stimulate [adult] stem cells to do the same thing,” he concludes.
In search of answers
Small stumbles in the developmental ballet of genes, even late in a pregnancy, can have far-reaching affects. That may have been the case with 9-month-old Sarah Myers. She was born with a heart that couldn’t push enough blood to her lungs, so surgeons had to rig a shunt between the two organs. The same genetic flaw underlying Sarah’s heart malformation caused other problems. At birth, she had blocked nostrils, which required the insertion of a breathing tube in her trachea. At 8 months old, Sarah was only as heavy as a big newborn.
Sarah’s mother, Lori Myers, estimates that her daughter has spent an average of 2 weeks out of every month in the hospital, a wrenching ordeal for the entire family. “After a while, you get tired of seeing people do this stuff to your child, even though you know it’s medically necessary,” she says.
Sarah’s pediatric cardiologist, Deepak Srivastava, is tired, also. His empathy for patients like Sarah, the anxiety of parents like Lori, and medicine’s frustrating ignorance about the causes of heart defects drove him from the clinic into the laboratory in search of answers.
“I got tired of having patients die and not being able to do anything about it,” Srivastava says. The first thing that a family asks when he tells them their child has a heart defect is, What caused it? “I think it would be very satisfying [for them] to understand why these things are happening,” he says.
In Sarah’s case, he was able to tell her parents immediately after her birth that her problems were genetic, because she shows an array of symptoms suggesting she has Charge’s syndrome, which can affect a child’s growth rate and the formation of the heart, eyes, ears, nose, and genitals. In the lab, Srivastava and his colleagues are now looking for the genetic signals that regulate the formation of the heart’s chambers and vessels and thus underlie the wide range of congenital heart defects.