Zap a layer of water with a strong electric field and, experiments dating back years suggest, some of the liquid freezes, even at comfortable, shirtsleeve temperatures. New experiments indicate that the electric field needn’t be so strong. If this result holds, it would indicate that warm ice could appear on a range of confining surfaces, including the minuscule crevices in ordinary rocks.
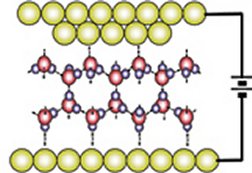
Warm-ice formation might also routinely affect molecular-scale activities such as protein behavior and the operation of nanoscale tips used for fine-scale lithography, say chemist Heon Kang of the Seoul (Korea) National University and his colleagues.
The team didn’t set out to investigate ice formation. “Our discovery was purely serendipitous,” Kang says.
The scientists were using a scanning tunneling microscope (STM)—an instrument with a sharp, electrically charged tip—to investigate how electrons move through a film of water to an underlying gold electrode (SN: 12/18&25/04, p. 394: An Electron Runs through It). As the STM’s gold tip vibrated up and down in the water, electrical readings indicated that the tip was hitting a barrier.
In the Aug. 19 Physical Review Letters, Kang’s team suggests what’s behind those readings. The researchers propose that a tiny blob of water quickly solidifies just beneath the descending tip. The smaller the distance between the tip and the electrode, the greater the strength of the electric field in the water between them. So, the field increased dramatically as the STM tip descended, the researchers explain.
At a so-called critical gap distance—as small as the height of two water molecules—the field probably coerces the water molecules into the regimented alignment of a solid. The exact process, however, remains obscure, the team notes.
The solid water stops the STM tip, Kang surmises. In tests during which the tip voltage was only about a hundredth of a volt, or 10 percent of the voltage that created a barrier, the tip was unimpeded.
According to calculations by the researchers, their thin films of water froze at field strengths of several million volts per meter. Large as that may seem, it’s only about a thousandth the field strength that computer simulations by other scientists had predicted would be necessary.
In fact, electric fields of 1 million volts per meter are downright pedestrian. Similar fields show up in many ordinary circumstances, such as when a person taking off a wool sweater makes static electric sparks. Such fields also turn up inside cell phone batteries and within ion channels in biological cells, Kang says.
Because of that ubiquity, these new findings could prove important, comments Miquel B. Salmeron of Lawrence Berkeley (Calif.) National Laboratory. Electric field–induced freezing in the folds of electrically charged biomolecules, such as proteins, may have a major impact on how those vital molecules behave.
Salmeron and other scientists note that the new evidence would be more convincing if it included a direct examination of the structure that the water molecules assumed under the tip.