Weighty Evidence
The link between obesity, metabolic hormones, and tumors brings the promise of new targets for cancer therapies
Living large can mean dying large, as familiar reminders about obesity’s link to cardiovascular disease and diabetes repeatedly emphasize. But those warnings often overshadow another threat from obesity: cancer. Excess weight accounts for 14 percent of cancer deaths in men, and 20 percent in women, researchers estimate. Among all preventable cancer risk factors, only smoking claims more lives.
Obesity’s link to cancer should come as no surprise. Signs of that relationship began to emerge 2 decades ago. In the late 1980s, laboratory researchers found connections between cancer and insulin—one of the major hormones that responds to obesity.
While the findings got little attention then, today at least a half-dozen companies are developing cancer drugs that interfere with the hormone’s cousin—insulinlike growth factor 1 (IGF-1).
“We’ve been working on this for 20 years,” says Derek LeRoith of the Mount Sinai School of Medicine in New York City. Yet until recently, “nobody ever bought into it.” After all, even if a tumor does need insulin, the rest of the body does too. The early research was seen as hardly relevant for disease treatment.
Not so today. If clinical trials find that dampening IGF-1 shrinks tumors in cancer patients, scientists will have not only a new kind of cancer drug but also a new source of insight into the interplay between body weight, metabolism, and cancer. In 2003, a study in the New England Journal of Medicine estimated that if the U.S. population were of a healthier weight, “90,000 deaths due to cancer could be prevented each year.” That number may not fall for generations, as obesity rates among even the youngest Americans continue to soar.
Heavy hormones
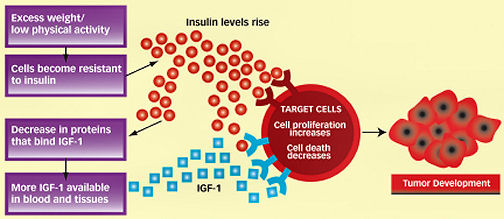
Lower weight and more physical activity can affect the production of insulin, the hormone that allows the body to soak up fuel. After a meal, food is broken down into glucose, which is the body’s main source of energy. Insulin triggers cells to take up and use glucose. As a person gains excess weight, the cells can become resistant to insulin’s actions. To compensate, the pancreas begins to produce more insulin, but it can’t stay in overdrive indefinitely. Eventually, insulin production will fall and blood glucose levels rise in some people.
The potent hormone IGF-1 and the related IGF-2 are very similar to insulin, helping support rapidly dividing cells, especially during childhood and adolescence. IGF-1 is a powerful driver of cell growth and body size: A toy poodle is a standard poodle with a faulty IGF-1 system.
The link between these insulinlike hormones and obesity is less clear than the connection between insulin and obesity. Although insulin and IGF-1 have individual parking places, or receptors, on a cell, some experiments suggest that at high enough levels, insulin starts to trespass on the IGF-1 receptor, LeRoith says.
In the late 1980s, laboratory researchers demonstrated that IGF-1 might have a role in cancer. Tumor cells were found to contain the IGF-1 receptor. In 1989, experiments with mice showed that blocking the receptor with an antibody could stop tumor growth. Researchers also found that mice bred to lack IGF-1 receptors in all their tissues were born tiny, thereby establishing the hormone’s significance in growth. More important for cancer research, cells taken from the miniature mice lacking IGF-1 receptors could not be transformed into tumor cells.
“A cancer cell has to have the IGF-1 receptor,” says Renato Baserga of the Kimmel Cancer Center at Thomas Jefferson University in Philadelphia, one of the field’s pioneers. “If not, it cannot grow.”
At first, results like these were puzzling. Unlike cancer genes that encode other proteins and start down the path to cancer after mutating, the IGF-1 receptor gene wasn’t altered in tumors. Also, IGF-1 receptors show up in normal tissues throughout the body. The hormone itself is such a basic substance for animal life that even flies produce it. It was hard to imagine that a normal receptor found in normal cells could have anything to do with cancer.
Then scientists had an idea. Malignant cells may be overly dependent on IGF-1 receptors, on a scale far surpassing the dependence of normal cells. A tumor is like a car—a gas-guzzling Hummer—with a stuck accelerator and no brakes. Even if IGF-1 doesn’t spark the ignition, the hormone keeps the gas tank full. Block IGF-1, according to this line of thinking, and the tumor suddenly finds itself running on empty.
Fueling cancer
Still, this notion might have stalled without two other developments. First, epidemiological studies began to find links between cancer and the insulin-IGF axis in people. Then, the entire field of cancer treatment underwent a transformation.
“What got people’s attention was the epidemiologic data,” says Doug Yee of the University of Minnesota Cancer Center in Minneapolis. In 1998, researchers reported in the journal Science that the risk of prostate cancer among men with the highest circulating levels of IGF-1 was four times as great as the risk among men with the lowest IGF-1 levels. Similar findings quickly followed in breast, colon, and other cancers.
So far, colon cancer has the most consistent association with insulin and IGF-1 levels, says Edward Giovannucci of the Harvard School of Public Health, a coauthor of the 1998 Science study. In 1999, he and his colleagues reported that colon cancer rates were more than twice as high among men who had the highest levels of IGF-1 as they were among men with the lowest IGF-1 levels.
Such findings fit with global patterns of the disease. “If you look at the rates of colon cancer across the world, populations where you expect people to have low insulin invariably have low rates of colon cancer,” Giovannucci says. Physical activity and reduced calorie intake can lower insulin levels; populations with more sedentary jobs and calorie-dense diets have higher rates of obesity and higher insulin levels.
“Once you become economically developed, colon cancer rates go up,” Giovannucci says. Also, the risks for colon cancer read largely like a list of red flags for type 2 diabetes. Diabetes itself is a risk factor for colon cancer.
Scientists are quick to point out that a higher insulin level isn’t the only chemical change that can occur with obesity. Levels of hormones that cause inflammation also rise, as do sex hormones, which can be produced in fat tissue. These and other changes in the body could themselves drive cancer. Or all these fluctuations could work in concert to feed malignancies.
And it might be not only the IGF-1 of middle age that matters, but also the IGF-1 production that orchestrates development early in life. Studies have suggested that babies born at the highest birth weights—and children experiencing early growth spurts—have a greater risk of cancer as adults.
While epidemiologists gathered evidence for a relationship between insulin and cancer, a second, unrelated advance gave the insulin-cancer connection new life: treatment success using antibodies that can attach to precise targets. Antibody-based drugs are large molecules that take the parking space so its rightful owner can’t use it. Herceptin, an antibody-based breast cancer treatment, came on the market in 1998, followed by others. Targeted antibodies were suddenly more than theory.
“I think once people got more comfortable making these drugs, the floodgates opened,” says Yee. And when pharmaceutical companies started casting for other promising targets for antibody development, the IGF-1 receptor suddenly looked attractive.
“They turned around and said, ‘You know, there’s this IGF receptor,'” says LeRoith of Mount Sinai. Drug development didn’t happen, and perhaps couldn’t have, until epidemiology and the technology caught up with the laboratory evidence.
Broad target
Nonetheless, an antibody that interferes with IGF-1 in people raises concerns. Although the full role of IGF-1 in adult tissues is still being worked out, rapidly growing tissues such as those in bone marrow and the intestine might become innocent by-standers of chemotherapy.
“You’re going to hit a receptor that’s present on every cell in the body, except the liver,” LeRoith says.
Also, in a case of molecular friendly fire, the drug might hit unintended targets. Because the insulin receptor and the IGF-1 receptor are cousins—they are actually more than 70 percent alike—some drugmakers worry about the possibility of accidentally interfering with the insulin receptor and making a cancer patient diabetic.
As an endocrinologist, LeRoith isn’t as disturbed by these scenarios as some of his colleagues may be. He believes chemotherapy-induced diabetes would be only temporary, and treatable. In the larger picture, it would not be as grave a threat as the cancer itself. Also, he says, chemotherapies already on the market cripple rapidly growing cells in the intestine, bone, and elsewhere. While these drugs do cause notorious side effects, the complications are generally accepted as the price of disease treatment.
So far, though, the experimental drugs haven’t caused major problems in early tests. Results of the first human-safety studies are starting to appear, most just in the past few months. The results are encouraging enough that companies are easing into larger studies.
“This was a target that was on everybody’s radar screen, but nobody jumped so strongly at it,” says Kapil Dhingra of Roche Pharmaceuticals in Nutley, N.J.
They have now. In October, at the International Conference on Molecular Targets and Cancer Therapeutics, researchers from Roche described a study of 34 patients with advanced tumors who received infusions of an experimental drug designed to target the action of IGF-1. Disease in nine patients stabilized. The most common side effects were fatigue, weight loss, and anorexia—complaints that are also often found in patients with advanced cancer. Subjects’ blood sugar levels appeared to remain stable.
The trial was designed to test the safety, and not the effectiveness, of the drug. But the researchers noted that it seemed to have a remarkable result in one of the study participants with Ewing’s sarcoma, a cancer of children and young adults that Yee in Minnesota had long ago identified as feeding heavily off IGF-1.
“We have a patient, a young woman in her 20s, who really has had honestly one of the best responses I’ve seen in 20 years,” says Razelle Kurzrock of the University of Texas M.D. Anderson Cancer Center in Houston. “When you see something like that in cancer, you’ve usually hit the molecular target.”
Within 6 weeks, the woman’s tumor melted away. The results were promising enough that Roche plans to test more patients.
The antibody-based drug that appears to be the farthest along in testing comes from Pfizer Inc., which has moved beyond safety studies into tests that gauge its effectiveness on cancer. Last summer, during a meeting of the American Society of Clinical Oncology, company researchers described results of a trial involving 70 patients with advanced lung cancer. About 46 percent of patients who received the drug in combination with standard chemotherapy improved, compared with 32 percent of those who did not get the anti-IGF-1 drug. Twenty percent of the patients getting the treatment experienced a jump in blood-glucose levels, implying some interaction with insulin. Later this year, the company hopes to report the effects of treatment on patient survival.
Other companies are also working on antitumor antibodies or on smaller molecules that will block the IGF-1 receptor. In the end, researchers say, the drugs may have a role in combination with standard treatments, and trials will probably also find that some tumors are more dependent than others on IGF-1.
“It’s not realistic to think that any one target is going to hit all of them,” says Kurzrock. Still, she says, “I would say this is going to be a good molecule.” If so, a line of research almost lost to the past could one day benefit cancer patients of the future.
Laura Beil is a freelance writer in Texas.