A weird type of zirconium soaks up neutrons like a sponge
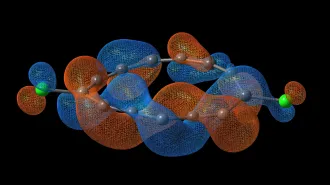
Scientists don’t yet know why zirconium-88 has such an affinity for the subatomic particles

NEUTRON HUNGRY A version of the element zirconium absorbs neutrons with a fervor unrivaled by almost any other type of known atom.
stockmorrison/Shutterstock