THE HYBRID FACTOR To understand signs of interbreeding among humans, Neandertals and other ancient hominids, scientists are studying physical changes in the bodies of various animal hybrids.
James Carey
Neandertals are the comeback kids of human evolution. A mere decade ago, the burly, jut-jawed crowd was known as a dead-end species that lost out to us, Homo sapiens.
But once geneticists began extracting Neandertal DNA from fossils and comparing it with DNA from present-day folks, the story changed. Long-gone Neandertals rode the double helix express back to evolutionary relevance as bits of their DNA turned up in the genomes of living people. A molecular window into interbreeding between Neandertals and ancient humans suddenly flung open.
Thanks to ancient hookups, between 20 and 35 percent of Neandertals’ genes live on in various combinations from one person to another. About 1.5 to 4 percent of DNA in modern-day non-Africans’ genomes comes from Neandertals, a population that died out around 40,000 years ago.
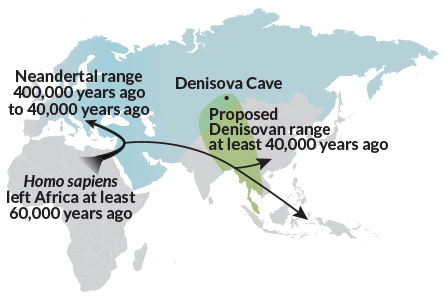
Even more surprising, H. sapiens’ Stone Age dalliances outside their own kind weren’t limited to Neandertals. Ancient DNA shows signs of interbreeding between now-extinct Neandertal relatives known as Denisovans and ancient humans. Denisovans’ DNA legacy still runs through native populations in Asia and the Oceanic islands. Between 1.9 and 3.4 percent of present-day Melanesians’ genes can be traced to Denisovans (SN Online: 3/17/16). Other DNA studies finger unknown, distant relatives of Denisovans as having interbred with ancestors of native Australians and Papuans (see “Single exodus from Africa gave rise to today’s non-Africans“). Genetic clues also suggest that Denisovans mated with European Neandertals.
These findings have renewed decades-old debates about the evolutionary relationship between humans and extinct members of our evolutionary family, collectively known as hominids. Conventional wisdom that ancient hominid species living at the same time never interbred or, if they did, produced infertile offspring no longer holds up.
But there is only so much that can be inferred from the handful of genomes that have been retrieved from Stone Age individuals so far. DNA from eons ago offers little insight into how well the offspring of cross-species flings survived and reproduced or what the children of, say, a Neandertal mother and a human father looked like.
Those who suspect that Neandertals and other Stone Age hominid species had a big evolutionary impact say that ancient DNA represents the first step to understanding the power of interbreeding in human evolution. But it’s not enough.
Accumulating evidence of the physical effects of interbreeding, or hybridization, in nonhuman animals may offer some answers. Skeletal studies of living hybrid offspring — for example, in wolves and monkeys — may tell scientists where to look for signs of interbreeding on ancient hominid fossils.
Scientists presented findings on hybridization’s physical effects in a variety of animals in April at the annual meeting of the American Association of Physical Anthropologists in Atlanta. Biological anthropologist Rebecca Ackermann of the University of Cape Town in South Africa co-organized the session to introduce researchers steeped in human evolution to the ins and outs of hybridization in animals and its potential for helping to identify signs of interbreeding on fossils typically regarded as either H. sapiens or Neandertals.
“I was astonished by the number of people who came up to me after the session and said that they hadn’t even thought about this issue before,” Ackermann says.
Streaming evolution
Interbreeding is no rare event. Genome comparisons have uncovered unexpectedly high levels of hybridization among related species of fungi, plants, rodents, birds, bears and baboons, to name a few. Species often don’t fit the traditional concept of populations that exist in a reproductive vacuum, where mating happens only between card-carrying species members.
Evolutionary biologists increasingly view species that have diverged from a common ancestor within the last few million years as being biologically alike enough to interbreed successfully and evolve as interconnected populations. These cross-species collaborations break from the metaphor of an evolutionary tree sprouting species on separate branches. Think instead of a braided stream, with related species flowing into and out of genetic exchanges, while still retaining their own distinctive looks and behaviors.
Research now suggests that hybridization sometimes ignites helpful evolutionary changes. An initial round of interbreeding — followed by hybrid offspring mating among themselves and with members of parent species — can result in animals with a far greater array of physical traits than observed in either original species. Physical variety in a population provides fuel for natural selection, the process by which individuals with genetic traits best suited to their environment tend to survive longer and produce more offspring.
Working in concert with natural selection and random genetic changes over time, hybridization influences evolution in other ways as well. Depending on available resources and climate shifts, among other factors, interbreeding may stimulate the merger of previously separate species or, conversely, prompt one of those species to die out while another carries on. The birth of new species also becomes possible. In hybrid zones where the ranges of related species overlap, interbreeding regularly occurs.
“Current evidence for hybridization in human evolution suggests not only that it was important, but that it was an essential creative force in the emergence of our species,” Ackermann says.
Hybrid faces
A vocal minority of researchers have argued for decades that signs of interbreeding with Neandertals appear in ancient human fossils. In their view, H. sapiens interbred with Asian and European Neandertals after leaving Africa at least 60,000 years ago (SN: 8/25/12, p. 22). They point to some Stone Age skeletons, widely regarded as H. sapiens, that display unusually thick bones and other Neandertal-like features.
Critics of that view counter that such fossils probably come from particularly stocky humans or individuals who happened to develop a few unusual traits. Interbreeding with Neandertals occurred too rarely to make a dent on human anatomy, the critics say.
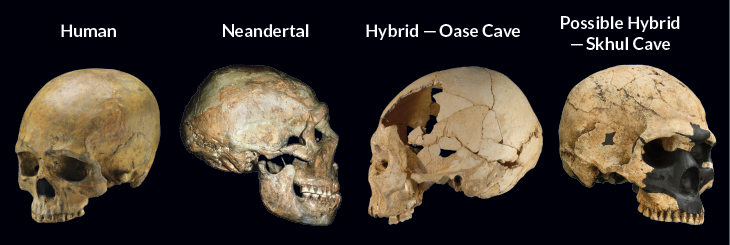
One proposed hybrid fossil has gained credibility because of ancient DNA (SN: 6/13/15, p. 11). A 37,000- to 42,000-year-old human jawbone found in Romania’s Oase Cave contains genetic fingerprints of a Neandertal ancestor that had lived only four to six generations earlier than the Oase individual.
Since the fossil’s discovery in 2002, paleoanthropologist Erik Trinkaus of Washington University in St. Louis has argued that it displays signs of Neandertal influence, including a wide jaw and large teeth that get bigger toward the back of the mouth. In other ways, such as a distinct chin and narrow, high-set nose, a skull later found in Oase Cave looks more like that of a late Stone Age human than a Neandertal.
Roughly 6 to 9 percent of DNA extracted from the Romanian jaw comes from Neandertals, the team found.
“That study gave me great happiness,” Ackermann says. Genetic evidence of hybridization finally appeared in a fossil that had already been proposed as an example of what happened when humans dallied with Neandertals.
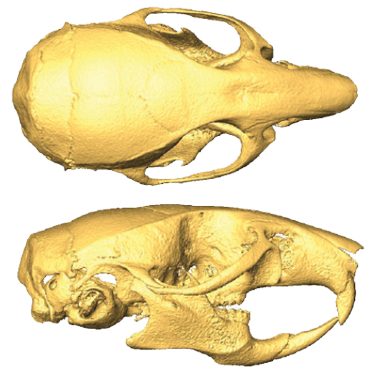
Hybridization clues such as those seen in the Oase fossil may dot the skulls of living animals as well. Skull changes in mouse hybrids, for instance, parallel those observed on the Romanian fossil, Ackermann’s Cape Town colleague Kerryn Warren reported at the anthropology meeting in April. Warren and her colleagues arranged laboratory liaisons between three closely related house mouse species.
First-generation mouse hybrids generally displayed larger heads and jaws and a greater variety of skull shapes than their purebred parents. In later generations, differences between hybrid and purebred mice began to blur. More than 80 percent of second-generation hybrids had head sizes and shapes that fell in between those of their hybrid parents and purebred grandparents. Ensuing generations, including offspring of hybrid-purebred matches, sported skulls that generally looked like those of a purebred species with a few traits borrowed from another species or a hybrid line. Borrowed traits by themselves offered no clear road map for retracing an animal’s hybrid pedigree.
Shape-shifting
Part of the reason for Ackermann’s caution stems from evidence that hybridization tends to loosen genetic constraints on how bodies develop. That’s the implication of studies among baboons, a primate viewed as a potential model for hybridization in human evolution.
Six species of African baboons currently interbreed in three known regions, or hybrid zones. These monkeys evolved over the last several million years in the same shifting habitats as African hominids. At least two baboon species have inherited nearly 25 percent of their DNA from a now-extinct baboon species that inhabited northern Africa, according to preliminary studies reported at the anthropology meeting by evolutionary biologist DietmarZinner of the German Primate Center in Göttingen.
Unusual arrangements of 32 bony landmarks on the braincase appear in second-generation baboon hybrids, Cape Town physical anthropologist Terrence Ritzman said in another meeting presentation. Such alterations indicate that interbreeding relaxes evolved biological limits on how skulls grow and take shape in baboon species, he concluded.
In line with that proposal, hybridization in baboons and many other animals results in smaller canine teeth and the rotation of other teeth in their sockets relative to parent species. Changes in the nasal cavity of baboons showed up as another telltale sign of hybridization in a recent study by Ackermann and Kaleigh Anne Eichel of the University of Waterloo, Canada.
The researchers examined 171 skulls from a captive population of yellow baboons, olive baboons and hybrid offspring of the two species. Skulls were collected when animals died of natural causes at a primate research center in San Antonio. Scientists there tracked the purebred or hybrid backgrounds of each animal.
First-generation hybrids from the Texas baboon facility, especially males, possessed larger nasal cavities with a greater variety of shapes, on average, than either parent species, Ackermann and Eichel reported in the May Journal of Human Evolution. Male hybrid baboons, in general, have large faces and boxy snouts.
Similarly, sizes and shapes of the mid-face vary greatly from one Eurasian fossil hominid group to another starting around 126,000 years ago, says paleoanthropologist Fred Smith of Illinois State University in Normal. Mating between humans and Neandertals could have produced at least some of those fossils, he says. One example: A shift toward smaller, humanlike facial features on Neandertal skulls from Croatia’s Vindija Cave. Neandertals lived there between 32,000 and 45,000 years ago. Smith has long argued that ancient humans interbred with Neandertals at Vindija Cave and elsewhere.
Ackermann agrees. Ancient human skulls with especially large nasal cavities and unusually shaped braincases actually represent human-Neandertal hybrids, she suggests. She points to fossils, dating to between 80,000 and 120,000 years ago, found at the Skhul and Qafzeh caves in Israel.
Some Neandertal genes eluded extinction, he suspects, because they were a help to humans. Several genetic studies suggest that present-day humans inherited genes from both Neandertals and Denisovans that assist in fighting infections (SN: 3/5/16, p. 18).
Helpful blends
One physical characteristic of hybridization in North American gray wolves is also a sign of interbreeding’s health benefits. Genetic exchanges with coyotes and dogs have helped wolves withstand diseases in new settings, says UCLA evolutionary biologist Robert Wayne.
“There are few examples of hybridization leading to new mammal species,” Wayne says. “It’s more common for hybridization to enhance a species’ ability to survive in certain environments.”
Despite their name, North American gray wolves often have black fur. Wayne and his colleagues reported in 2009 that black coat color in North American wolves stems from a gene variant that evolved in dogs. Interbreeding with Native American dogs led to the spread of that gene among gray wolves, the researchers proposed. The wolves kept their species identity, but their coats darkened with health benefits, the scientists suspect. Rather than offer camouflage in dark forests, the black-coat gene appears to come with resistance to disease, Wayne said at the anthropology meeting. Black wolves survive distemper and mange better than their gray-haired counterparts, he said.
Similarly, DNA comparisons indicate that Tibetan gray wolves acquired a gene that helps them survive at high altitudes by interbreeding with mastiffs that are native to lofty northern Asian locales. Intriguingly, genetic evidence also suggests that present-day Tibetans inherited a high-altitude gene from Denisovans or a closely related ancient population that lived in northeast Asia.

Making contact
Like wolves, ancient hominids were medium-sized mammals that traveled great distances. It’s possible that an ability to roam enabled humans, Neandertals and Denisovans to cross paths in more populated areas, resulting in hybrid zones, paleoanthropologist John Hawks of the University of Wisconsin–Madison suggests.
Hominids may have evolved traits suited to particular climates or regions. If so, populations may have rapidly dispersed when their home areas underwent dramatic temperature and habitat changes.
Instead of slowly moving across the landscape and stopping at many points along the way, hominid groups could have trekked a long way before establishing camps in areas where other hominids had long hunted and foraged. Perhaps these camps served as beachheads from which newcomers ventured out to meet and mate with the natives, Hawks says.
All ancient hominid populations were genetically alike enough, based on ancient DNA studies, to have been capable of interbreeding, Hawks said at the anthropology meeting. Specific parts of Asia and Europe could have periodically become contact areas for humans, Neandertals, Denisovans and other hominids. Beneficial genes would have passed back and forth, and then into future generations.
Ackermann sees merit in that proposal. Hominid hybrid territories would have hosted cultural as well as genetic exchanges among populations, she says, leading to new tool-making styles, social rituals and other innovations.
“These weren’t necessarily friendly exchanges,” Ackermann says. Many historical examples describe cultural exchange involving populations that succumb to invaders but end up transforming their conquerors’ way of life.
However genes, behaviors and beliefs got divvied up in the Stone Age, a mix of regional populations — including Neandertals and Denisovans — can be considered human ancestors, she theorizes. They all contributed to human evolution’s braided stream.
That’s a controversial view. Neandertals and Denisovans lived in relatively isolated areas where contact with other hominid populations was probably rare, says paleoanthropologist Matthew Tocheri of Lakehead University in Thunder Bay, Canada. Random DNA alterations, leading to the spread of genes that happened to promote survival in specific environments, played far more important roles in human evolution than occasional hybridization did, Tocheri predicts.
Neandertals and Denisovans can’t yet boast of being undisputed hybrid powers behind humankind’s rise. But a gallery of interbreeding animals could well help detect hybrid hominids hiding in plain sight in the fossil record.
This article appears in the October 15, 2016, issue of Science News with the headline, “The Hybrid Factor: The physical efffects of interbreeding among animals may offer clues to Neandertals’ genetic mark on humans.”
Editor’s Note: This article was corrected on October 12, 2016, to note Fred Smith’s current university affiliation.