
KISSING ANCIENT COUSINS Interbreeding between Neandertals and early modern humans has left a genetic mark on non-Africans that may affect their health.
H. Neumann/Neanderthal Museum
- More than 2 years ago
Finding Neandertal ancestors in the human family tree was shocking enough when researchers announced it in 2010. Now the implications for modern-day people carrying surviving Neandertal DNA may prove just as stunning.
Today, Europeans and Asians carry, on average, between 1.5 percent and 4 percent Neandertal DNA. A flurry of new studies suggests that the genetic hand-me-downs may once have helped human newcomers adjust to their new homes. But these genetic bits and pieces may no longer be helpful, and may even raise the risk of depression, heart disease, some skin conditions, allergies and other maladies.
“There was, and still is, a lingering cost of having this admixture,” says John Capra, an evolutionary geneticist at Vanderbilt University in Nashville.
Capra and colleagues examined DNA and electronic health records from more than 28,000 people of European descent to determine whether genes from extinct ancestors were associated with diseases. Genetic variants inherited from Neandertals were associated with a slightly increased risk of depression and heart attack as well as corns and callouses and other skin disorders, the researchers reported February 11 at the American Association for the Advancement of Science’s annual meeting in Washington, D.C., and in the Feb. 12 Science.
For instance, Neandertal variants account for about 1 percent of the genetic risk of getting depression. Interestingly, some bits of Neandertal DNA actually seem to protect against depression, study coauthor Corinne Simonti, also of Vanderbilt University, said at a press briefing at the AAAS meeting. So whether a person is depressed may depend, in part, on which bits of Neandertal DNA they inherited.
Other researchers caution that Capra’s group hasn’t demonstrated that the Neandertal variants are the genetic changes responsible for the disease. They may be innocent bystanders implicated just by their proximity to variants that are really causing problems. And, says evolutionary geneticist Rasmus Nielsen of the University of California, Berkeley, “because we’re talking about genetic variation here, the effect is not in one direction — so the study doesn’t show that you’re more (or less) likely to become ill because of Neandertal DNA.”
Story continues after map
Inheriting immunity
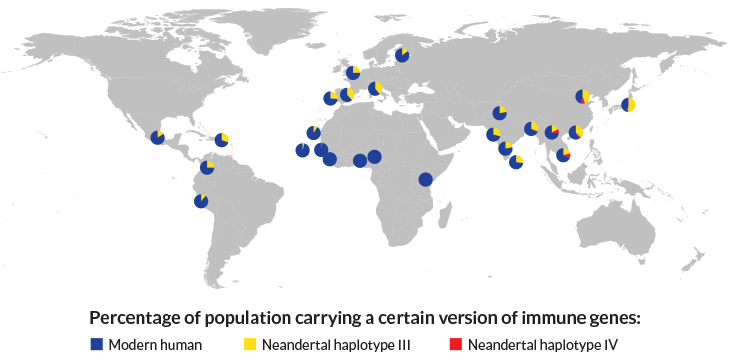
Present-day people participating in the 1000 Genomes project inherited one of three versions, or haplotypes, of immunity genes. Neandertals passed haplotype III to all non-Africans, and some Asians also inherited Neandertal haplotype IV. Those versions of the immunity genes may have helped human ancestors fend off new pathogens, but now contribute to allergies.
If Neandertal DNA is harmful, why is it still hanging around? It may not always have been a deficit, says Capra. His group found that Neandertal DNA is associated with a disorder in which the blood clots too much due to increased activity of a gene called SELP. It may have been helpful to inherit the Neandertal gene in the Stone Age when closing a wound quickly was a major concern, Capra says. “It’s certainly possible that some of these Neandertal contributions were beneficial 40,000 years ago, but are not now.”
No one knows exactly when human-Neandertal interbreeding occurred or how much went on. Estimates range from around 60,000 years ago or more until shortly before Neandertals disappeared about 30,000 years ago (SN: 6/13/15, p. 11). Some studies have concluded that Asians’ ancestors mixed with Neandertals more than once, leaving Asians with more Neandertal DNA than Europeans (SN Online: 2/12/15). In addition, Asians also inherited a small amount of DNA from another extinct group of human relatives called Denisovans.
That genetic legacy was not passed along wholesale like a precious Ming vase handed down through generations. Instead, DNA was shuffled during reproduction. Families bequeathed different bits of DNA to their heirs, as if each child inherited different shards of a shattered vase. If everyone’s Neandertal genetic keepsakes were glued back together, there would be enough pieces to reconstruct 20 percent or more of a Neandertal’s genome.
“In a way, Neandertals survived in us,” says Fernando Racimo, a population geneticist at the University of California, Berkeley. “You could say that they’re not really completely extinct.”
Some parts of the human genome don’t contain any Neandertal DNA at all, while other parts may be 60 percent or more Neandertal (SN: 3/8/14, p. 12). Scientists have debated whether that means that evolution has favored some Neandertal genes because they benefit humans or that evolution has been slowly weeding out Neandertal DNA, and some genes have risen to prominence by chance.
Early on, natural selection may have played a numbers game in determining what genes would stay or go. In independent studies published online last fall at bioRxiv.org, two groups of researchers argue that dwindling Neandertal populations had built up weakly harmful mutations. Because the Neandertal populations were small and inbred, natural selection couldn’t efficiently clear out the mutations. All those small genetic disadvantages would have added up to make the average Neandertal at least 40 percent less evolutionary fit than the average early modern human fresh from Africa, Nielsen and evolutionary geneticist Kelley Harris of Stanford University, calculate in a report published October 31.
When humans and Neandertals interbred, the humans’ larger population size allowed evolution to weed out the slightly bad variants, a group of researchers from the University of California, Davis reports in a November 21 paper. Both groups agree that the burden of extra Neandertal mutations would have been a drag on early human-Neandertal hybrids. Europeans and Asians are still shouldering that mutational load today. Non-Africans are about 0.5 percent less evolutionarily fit than their African counterparts thanks to Neandertal DNA, Harris and Nielsen say.
On the whole, the researchers argue, Neandertals’ extremely narrow population bottleneck was bad for them and their modern human descendants. But a wealth of genetic data indicate that at least some genes inherited from humans’ extinct cousins were evolutionary windfalls. For instance, a gene Tibetans inherited from Denisovans allowed them to adapt to high altitudes (SN: 8/9/14, p. 8).
Some of the most Neandertal-rich regions of the modern human genome are those that contain immune system genes. There’s a good reason for that. “Of all the challenges, forces and threats humans have faced over the course of evolution, pathogens have been one of the greatest,” says Lluis Quintana-Murci, a population geneticist at the Pasteur Institute in Paris.
Genes involved in the body’s first line of defense against pathogens are under strong evolutionary pressure not to change, Quintana-Murci and colleagues report in the Jan. 7 American Journal of Human Genetics. Some of the genes under the strongest pressure are Toll-like receptor genes TLR1, TLR6 and TLR10. Mutations in those genes, which help detect pathogens and coordinate inflammatory responses, could lead to severe, life-threatening diseases, he says.
But there’s a conundrum: In modern humans, these genes would have had to change to deal with new pathogens that humans encountered outside of Africa. It would have taken humans thousands of years to build up the right mutations. Interbreeding with Neandertals may have provided a shortcut to immunity without risking life-threatening mutations, Quintana-Murci surmises. Neandertals had been living with European pathogens for hundreds of thousands of years, gradually accumulating helpful tweaks to ward off the pathogens, but to not overreact and produce strong inflammation that could kill an infected person, he says. Those TLR genes were some of those most often inherited from Neandertals, the researchers found.
“Perhaps spending a night or two with a Neandertal was a small price to pay for getting thousands of years of genetic adaptation,” Capra said at the AAAS press briefing.
Computational biologist Janet Kelso of the Max Planck Institute for Evolutionary Anthropology in Leipzig, Germany, and colleagues also examined the same TLR genes. They report, also in the Jan. 7 American Journal of Human Genetics, that humans had inherited at least three versions from extinct ancestors.
One Neandertal version of those genes, called core haplotype III, was found in all the non-African populations the researchers examined. It was also found in two people from Northwest Gambia, but not in other Africans. A second Neandertal version of the genes, core haplotype IV, was also found in Asians. That result supports earlier work suggesting that Asians’ ancestors interbred more than once with Neandertals. “We can be pretty sure that this was not a one-off event,” Kelso says. A Denisovan version of the genes, core haplotype VII, was found in two Southeast Asians.
Kelso’s group took a closer look at what the ancient genes are doing for present-day people. The Neandertal versions don’t change the TLR genes themselves, but change activity of the genes, Kelso’s group discovered. The TLR genes are more active in people with Neandertal core haplotype III. Those people are less likely to get infected with the ulcer-causing Helicobacter pylori bacterium. But the advantage comes with a cost: Those people are more prone to allergies, the researchers found.
“We can’t really blame Neandertals for all the diseases we have,” Capra said, but, on the whole, the ancient DNA probably isn’t doing modern people much good.






