Personalized gene editing saved a baby, but the tech’s future is uncertain
Costs, government regulation and lack of know-how could hinder wider use of the CRISPR tech

Thanks to a gene therapy designed just for him, KJ Muldoon (center) may soon leave the hospital to join his siblings (pictured) at home.
CHOP
Editor’s note: On June 3, 2025, KJ Muldoon was discharged from the hospital and is now at home with his family.
When a baby born in Philadelphia was announced as the first person to get a gene therapy designed just for him, many people hailed the achievement as a starting point to treat virtually any genetic disease.
But there is a long road that researchers and regulators need to pave before other people with genetic disorders can get bespoke gene therapies.
Here’s what you need to know about this personalized therapy and how it may affect gene therapy moving forward.
What led to this pioneering gene editing?
On May 15, doctors and researchers at Children’s Hospital of Philadelphia (CHOP) and colleagues described the personalized gene therapy in the New England Journal of Medicine. The treated child, KJ Muldoon, has a disorder that prevents his liver from converting ammonia from broken-up proteins to urea. Urea is flushed from the body in urine.
KJ’s form of the disease stems from a mutation in both copies of his CPS1 gene. That gene contains instructions for building an enzyme called carbamoyl-phosphate synthetase 1 that is important in the urea cycle — the conversion of ammonia to urea. Without the enzyme, ammonia levels shoot up and can cause brain and nerve damage and death. This deficiency affects about 1 in 1.3 million people, about half of whom die in early infancy. Low protein diets, medications that help lower ammonia levels and ultimately liver transplants are used to treat the condition, though these measures may not entirely cure the disorder.
KJ was born prematurely in August and was too small for a liver transplant. Medications and extremely low protein diets helped keep ammonia levels in his blood down. But the levels often spiked, and doctors worried he could be left with permanent brain damage or die.
Cardiologist Kiran Musunuru of the University of Pennsylvania Perelman School of Medicine and pediatrician and medical geneticist Rebecca Ahrens-Nicklas of CHOP had already been practicing for such a scenario. They quickly assembled a coalition of academic and industry scientists to manufacture the gene therapy and make sure it was safe to give to a person, Musunuru said in a news briefing May 12.
The team also applied to the U.S. Food and Drug Administration for permission to treat the baby. The FDA recognized that “KJ was very, very sick and there wasn’t time for business as usual,” Musunuru said. The agency approved the treatment within one week of getting the application.
KJ has gotten three infusions of his personalized gene therapy. He isn’t cured — it’s not known whether all the cells in his liver have the corrective edits. Even some patients with liver transplants can have ammonia spikes after infections. But KJ can eat more protein and needs much less medication to keep his ammonia levels in check, Ahrens-Nicklas said.
What is the personalized gene therapy?
KJ’s gene therapy is based on CRISPR, a targeted gene-editing system which is being developed to treat cancers and a wide variety of genetic diseases. This child got a molecular pencil version of CRISPR called a base editor. That editor chemically erased a mutation in KJ’s broken CPS1 gene and wrote in the correct DNA letter, or base. Base editors are being used as possible treatments for high cholesterol and other conditions. (Musunuru is a founder of the company developing the cholesterol gene therapy.)
This therapy started with messenger RNA, or mRNA, instructions for making the base editor. Messenger RNA is a go-between molecule, a copy of DNA instructions for building a protein. The mRNA is read by cellular machinery and used to produce the protein, which then carries out a particular job. In this case, making the base editor that would correct the typo in KJ’s gene.
The researchers encased mRNA instructions for making the base editor in a lipid nanoparticle, which is “basically like a soap bubble,” says Petros Giannikopoulos, a molecular pathologist at the University of California, Berkeley’s Innovative Genomics Institute. Giannikopoulos was involved in testing KJ’s base editor to make sure it didn’t cause unintended changes elsewhere in his DNA. COVID mRNA vaccines made by Moderna and Pfizer-BioNTech use similar lipid nanoparticle technology to deliver mRNA to cells. In KJ’s case, the nanoparticles specifically directed the therapy to the liver.
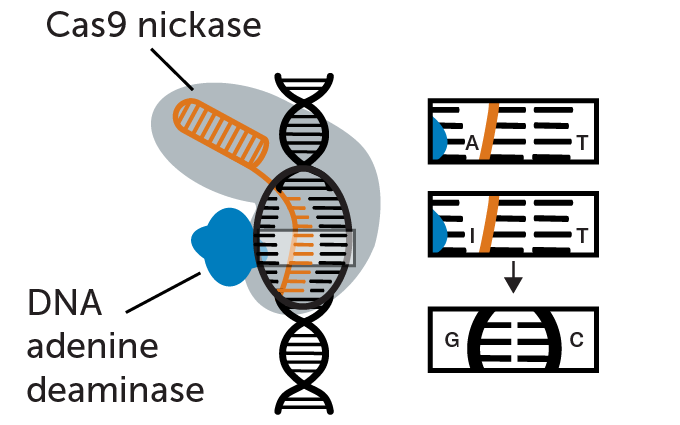
Fixing a DNA typo
Researchers used a CRISPR base editor to repair a typo in one baby’s gene, in this case changing an incorrect adenine (A) to the correct guanine (G). A guide RNA (orange) shepherds the base editor to the typo. The base editor is a version of a DNA-cutting enzyme called CRISPR/Cas9 that has been altered so that it nicks one of two DNA strands at the typo (therefore, called Cas9 nickase). Tacked on to the Cas9 nickase is another enzyme called a DNA adenine deaminase. It chemically converts the DNA base adenine (A) to inosine (I). Inosine isn’t a usual DNA building block so DNA repair enzymes in a cell convert it to guanine (G), fixing the typo.
Researchers tested the base editor in lab-grown cells in a dish. They then gave doses of the therapy to crab-eating macaques to test for toxicity. The team also genetically engineered mice to carry KJ’s mutation, then used the base editor to correct the DNA typo. It took only six months to develop and test the therapy.
KJ got his first intravenous infusion containing the therapy in February. He got a very low dose to start with. Two subsequent doses have been higher. The researchers will continue to monitor KJ, and he soon may be able to leave the hospital where he has lived since birth.
What makes KJ’s case special?
“What was very unique about this was that the therapy was manufactured for an individual patient,” Giannikopoulos says. “This child had a specific mutation…. The therapy was designed for that specific mutation. That was why this was so monumental.”
Previous CRISPR and other gene therapies, including one approved by the FDA to treat sickle cell disease and beta-thalassemia, are proactive, he says. That means “we make and preapprove something, and then wait for the patients to come along” whose mutations match the therapy. KJ’s treatment, in contrast, is reactive. “The patient was diagnosed. We sequenced and found the defect in the genome. And then we designed and manufactured and did all the work to target that specific mutation.”
That personalized approach could be used for many people with super rare diseases.
“I don’t think I’m exaggerating when I say that this is the future of medicine,” Musunuru said in the news briefing. “This is a step towards the use of genetic editing therapies to treat a wide variety of rare genetic disorders for which there are currently no definitive medical treatments.”
Giannikopoulos agrees. “What happened at CHOP was basically the birth of a new medical subspecialty,” he says.

Doctors Kiran Musunuru (left) and Rebecca Ahrens-Nicklas (right, holding KJ) had been practicing to make bespoke gene therapies when KJ was born with a super rare genetic disease. In six months, the researchers and colleagues created and tested the gene therapy used to treat KJ.
CHOPCan every genetic disease be treated this way?
Probably not all of them, Giannikopoulos says. There are more than 7,000 known genetic diseases. He estimates that 15 to 20 percent of those might be fixable using currently available gene-editing technology. Ones that are caused by single letter typos in a single gene might be correctable using a base editor.
Other CRISPR editors, including a very versatile version called a prime editor, potentially can repair many types of mutations, including small deletions. Cambridge, Mass.–based Prime Medicine announced in a news release May 19 that it had successfully treated a person with a different rare disease using prime editing.
Scientists must also be able to easily get the editor where it needs to go. Diseases that affect easily accessible organs may be the most treatable, Giannikopoulos says. Immune system or blood disorders may be fixed by removing stem cells from the bone marrow, editing them in the lab and then returning them to the patient. Other organs such as the skin, eye or liver (as in KJ’s case) are relatively easy to deliver gene therapy to inside the body. Other gene therapies delivered inside the body include an approved treatment for Duchenne muscular dystrophy.
But diseases that affect multiple organs at once or those that affect hard-to-reach organs might not be easily treated. For instance, “we’re not very good at targeting the kidney right now,” Giannikopoulos says.
When to deploy gene therapy also matters. “For some diseases, you might need to intervene, maybe in utero, but we haven’t gotten there yet.”
“You’ve also got to understand the disease well enough to be able to intervene. Because [for] some diseases, we know what the mutation is, but we don’t really understand how the mutation is causing trouble.”
What else is needed for personalized gene therapy to become widespread?
Changes in regulations would probably be necessary, Giannikopoulos says. Currently, gene therapies are usually approved for correcting a specific mutation. But some genes may have hundreds of disease-causing mutations, so only a subset of people with a particular disease may be eligible.
Instead, Giannikopoulos and other researchers argue that the general procedure and materials, including the delivery vehicle and gene editor, should be treated as a platform or umbrella therapy. That umbrella intervention would be tested for safety and efficacy and then could be deployed as needed for a patient’s particular mutation. “Otherwise, if everything needs to be repeated in terms of the safety and efficacy for every one of these tens of thousands or potentially hundreds of thousands of specific mutations, then we’ll never get there,” Giannikopoulos says.
It may also be a struggle to get insurance to pay for expensive one-off gene therapies, he says. Funds have been a common and concerning theme for gene therapy. Even when therapies have been proven to work, companies often don’t have the resources to conduct clinical trials to get FDA approval, or to keep the treatments on the market once they have been approved. For instance, Prime Medicine is no longer developing its just-announced gene therapy.
Drastic cuts in research funding may also hinder early gene therapy development in academic laboratories in the United States.
Standardized playbooks for designing and implementing gene therapies are also needed because many doctors want to treat patients with genetic diseases, but don’t have the know-how, Giannikopoulos says. He and Musunuru are part of the U. S. National Institutes of Health–funded Somatic Cell Genome Editing Consortium that is putting together such playbooks. “That’s going to be really how to scale this, [by] teaching everybody how to fish around the world.”