By the time pharmaceutical giant Merck yanked its painkiller Vioxx from the market last fall, the evidence had become overwhelming that the pills were nearly doubling patients’ chances of heart attacks or strokes.
What’s particularly disturbing is that the drug before its 1999 approval had passed the full battery of required animal and human tests. The Vioxx incident (SN: 10/30/04, p. 286: Available to subscribers at COX-2 inhibitor pulled off market) and growing uncertainty about the safety of other approved medications such as Celebrex and naproxen are highlighting inadequacies of current testing methods. If time-honored testing on laboratory animals and groups of people isn’t enough, maybe biomedical specialists should think outside of the box, some scientists have reasoned.
Enter the animal-on-a-chip.
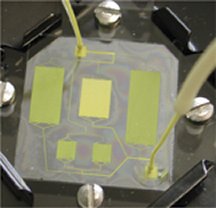
It’s a silicon or plastic chip with compartments containing cells of this organ here and that organ there. The cells can come from the same animal or from different species, thereby creating a chip version of Frankenstein’s monster.
The device also contains channels to circulate fluid, as if it were blood, among those miniorgans. Researchers inject whatever chemical compound they wish to study into the chip’s fluidic system, and the high-tech guinea pig simulates the responses of an organism, real or composite.
More complex than conventional cell cultures, the devices can better replicate a living creature’s bodily reactions. Yet because they’re far simpler than actual animals, the chips may make harmful physiological processes easier to spot—and to understand.
“Our idea is to bridge between cell-based assays and animal studies,” says Gregory T. Baxter of Hµrel Corp., a Beverly Hills, Calif.–based company that he cofounded in 2003 to develop and sell animal-on-a-chip devices.
Recently, researchers at Cornell University reported a proof-of-principle demonstration that such devices can replicate responses of intact animals to toxic chemicals. Buoyed by this success, the animal-on-a-chip approach is attracting interest from entrepreneurs and pharmaceutical companies.
Baxter says that the pharmaceutical scientists he has canvassed have been enthusiastic about tailoring animal-on-a-chip devices to their needs.
The technology “looks very promising,” says Peter G. Lord, who leads studies of drug-toxicity mechanisms for Johnson & Johnson Pharmaceutical Research and Development in Raritan, N.J.
Bigger isn’t better
There’s no perfect way to predict a particular chemical’s effects on the human body. The most common technique—exposing cells growing in a laboratory to the agent of interest—also tends to be the least informative. Cells in conventional lab cultures—usually a single cell layer at the bottom of a petri dish—rarely retain many native functions.
“Cells removed from the body are often not authentic in their responses,” says Cornell bioengineer Michael L. Shuler, who serves on Hµrel’s board. Consequently, cell-culture tests usually offer no clue to physiological effects that could take a drug candidate out of the running downstream in the product-development pipeline.
“Over 90 percent of the compounds that go into animal studies fail,” Baxter says.
Animal tests provide more make-or-break clues regarding drug candidates than do cell-based assays. But critical differences in physiology and biochemistry among animals create uncertainty about how well the findings apply to people. Moreover, some people have ethical objections to the use of animals in biomedical tests, in general.
Human trials bring their own complexities. Besides sometimes posing ethics questions, they’re time-consuming and costly. “Human clinical trials can cost more than $100 million,” Shuler says. “If you can spot the compound that’ll fail in [final-phase] clinical trials . . . that’s where you save money.”
Testing potential drugs on people sometimes misses harmful effects. The population that ends up taking a popular drug is far larger than the group of participants in a trial and may continue taking an approved drug for decades. Besides Vioxx, other drugs that were taken off the market after their initial approval include allergy-relieving Seldane, antidiabetes Rezulin, and the cholesterol-lowering Baycol.
Besides offering a new testing regime intermediate between cell cultures and intact organisms, animal-on-a-chip tests could provide data that render those other categories of testing more precise and effective, the chips’ developers say.
By means of such chips, Shuler says, “one can find the compounds and conditions most likely to give interesting results in animals.” Because that could minimize the number of animal tests needed, “we look at this as an animal-sparing technology,” he adds.
At the same time, because experimentation on chips can be automated, testers could carry out the on-chip tests at a rapid clip.
Shuler began pursuing this alternative approach in the late 1980s. Back then, the technology could have been called “animal-on-a-benchtop” because that’s how much space was needed for the pumps, fluid reservoirs, and milk-bottle-size flasks of cells that Shuler and his team interconnected with surgical tubing. For example, he put liver cells in one bottle and lung cells in another, then used pumps, which served somewhat like a heart, to force bloodlike fluid through the bottles.
Initially, Shuler wasn’t seeking an alternative to animals. He had a more esoteric goal: physical analogs for a type of mathematical models that engineers use to represent physiological processes.
In 1997, molecular biologist Baxter joined the Cornell faculty. He had just finished a stint in industry, where he had been developing microfluidics systems—miniature fluid-manipulating devices—for testing drugs on cell cultures.
Baxter recalls that when, just a few days after arriving on campus, he first met Shuler, “we immediately saw a fit for miniaturized systems to make [Shuler’s setups] more physiologically realistic.”
Chip off the old body
Shuler coined the term animal-on-a-chip in 1999, when he, Baxter, and their colleagues were devising microversions of Shuler’s tabletop setups. All the compartments fit on a postage stamp–size piece of silicon sandwiched between layers of Plexiglas.
Although each compartment contains cells growing as if in cell culture, the flow of fluids between the chambers makes the system more realistically represent an animal. Baxter left Cornell in 2001 to commercialize animal-on-a-chip technology.
After more years of fiddling, Shuler’s group finally built a four-chamber, chip-size system with which they recently demonstrated the known harmful effects of naphthalene, the main ingredient of mothballs, on various tissues.
The chip contained liver cells to metabolize the compound, fat cells to absorb chemicals, and lung cells, which are susceptible to naphthalene damage.
In both lung and liver cells, naphthalene reduced the concentration of a protective compound, glutathione, which chemically combines with some of naphthalene’s metabolites, the researchers reported in the February 2004 Biotechnology Progress. That work demonstrated that the system could replicate known effects of a well-studied toxic chemical.
In another proof of principle, Hµrel scientists tested the anticancer drug Tegafur using an animal-on-a-chip setup, says Baxter. In the February 2004 American Biotechnology Laboratory newsletter, he and Hµrel cofounder Robert M. Freedman describe tests showing that Tegafur becomes a potent anti–colon cancer agent when activated by the chip’s liver cells.
In contrast, the drug had no effect on colon-cancer cells exposed directly to it in cell cultures because those lacked the liver cells that activate the compound.
In a new project based on a “human-on-a-chip”—the first chip by the Cornell team to use only cells from people—Shuler and his colleagues are investigating the response of uterine tumors to chemotherapy regimens. Often, after a seemingly successful round of chemotherapy, mutated tumor cells roar back as deadly, drug-resistant cancers.
Although there are compounds known to kill those resurgent cancers, the substances can’t be given as therapies because they would kill the patient at the doses required. However, there might be safe combinations of lower doses of such anticancer agents. Finding such a therapeutic window is the goal of the uterine human-on-a-chip, Shuler says.
Get real
Still at an early stage, animal-on-a-chip devices so far offer only crude analogies for the organisms they’re intended to emulate. Some scientists are figuring out ways to make the cells in a compartment of a microfluidic setup more closely resemble an intact organ.
For example, Linda G. Griffith of the Massachusetts Institute of Technology (MIT) and her coworkers have devised a “bioreactor” containing a fingernail-size patch of silicon penetrated by hair-thin holes. Inside those holes, liver cells of multiple types form hollow configurations similar to those found in the liver itself.
Likewise, Martin L. Yarmush of Rutgers University Department of Biomedical Engineering in Piscataway, N.J. and his colleagues have devised a microfabrication technique for depositing two types of liver cells onto glass in geometrical arrangements that simulate cell arrangements in the actual organ. For instance, strips of one cell type can alternate with strips of the other.
In both that microenvironment and the one devised at MIT, liver cells retain more of their natural functions than do such cells in ordinary cell cultures, the teams have reported.
Shuichi Takayama of the University of Michigan at Ann Arbor is taking on another central facet of physiology in his chip designs. He and his coworkers recently unveiled a new type of microfluidics system that they say can be easily configured to simultaneously create different physiologically relevant conditions, such as flow rates and fluid-induced forces, in different regions of the chip.
Made of soft poly(dimethylsiloxane) plastic, the postcard-size prototype consists of a half-dozen compartments and interconnecting channels. It rests on an array of more than 300 computer-actuated metal pins, each about a millimeter in diameter.
As pins push upward, they pinch off specific channels. Sets of three adjacent pins on any channel can pump fluid when each pin is triggered in succession. The apparatus is easy to build, Takayama says, because the pin array is an off-the-shelf device—an automated Braille display for blind readers.
In the Nov. 9, 2004 Proceedings of the National Academy of Sciences, the Michigan researchers report manipulating fluid flowing through their novel apparatus in a way that deposited immature muscle cells into each of the device’s compartments. For 3 weeks, the computer then controlled fluid circulation so that each chamber received saline solution at a different rate. This resulted in varied cell morphologies and growth patterns.
To make animals-on-chips even more useful, researchers are adding digestion to the package. In collaboration with U.S. Department of Agriculture physiologist Raymond P. Glahn, Shuler and his colleagues are building a chip-scale gastrointestinal tract.
Because scientists working with other chips add the compound to be tested directly to the circulating fluid, they explore the effects of “what you might call a shot or an IV [intravenous administration],” Shuler says. However, he adds, “with the GI tract, we can ask what would happen if you gave a pill or a liquid.”
With the coming improvements, animal-on-a-chip devices may someday be so true to life that they could open the door to a new type of personalized medicine. Call it “you-on-a-chip.”
For instance, whereas a doctor today routinely biopsies tissue suspected of being malignant, physicians of the future might also take small samples of healthy tissues, Shuler says.
If the suspicious tissue turns out to be cancerous, an oncologist would then put all the samples from the patient into compartments of a microfluidic chip. Then, trial runs of various chemotherapy agents could indicate which protocol would be most effective against the cancer yet least harmful to that particular patient’s healthy tissues.
You-on-a-chip could be a lifesaver.





