About 2,000 years ago, the Roman geographer Strabo wrote about the residents of Latakia, Syria, who rowed their boats 4 kilometers out into the salty Mediterranean, dove a few meters to the ocean floor, and collected fresh drinking water in goatskin containers for their city. No miracle, this—marine boaters could do the same today at a spot about 10 km east of Jacksonville, Fla. In fact, similar freshwater springs erupt on the seafloor near many shores. These flows of water originate on land and end up in the sea, just as rivers do—only they take a subterranean route to their destinations.
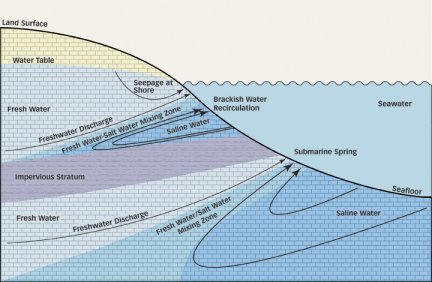
Such underground rivers form only under certain geologic conditions. At some sites, the water flows from onshore aquifers to the sea through porous rocks and then seeps up through the seafloor. At many more locales, groundwater drains at low tide through sand or other porous shoreline sediments into the ocean.
Collectively called submarine groundwater discharge, such flows to the sea are gaining increasing attention in scientific circles. Their flow rates are often low—sometimes just a few liters per day for each square meter of seafloor—but those trickles become significant when tallied over large areas. That influx of fresh water alters the ocean’s salinity near the seafloor, a factor that influences the makeup of the ecosystems in those places.
Many of these ecosystems are threatened by increasing amounts of nutrients or pollutants in the water arriving from the shore. Those substances can have widespread effects, fueling algal blooms and microbial growth in sediments or smothering coral reefs.
Few, if any, people now take advantage of submarine springs—it’s far too easy today to drill a hole on land to reach fresh water. But as scientists become more aware of the large volume of submarine groundwater discharge in some locations, they’re figuring out how to detect and measure the phenomenon. They’re also constructing theoretical models of how it works and developing devices that can accurately measure gases and nutrients dissolved in the water.
No-man’s-land
Although submarine groundwater discharges have been known for centuries, they’ve often been considered just local curiosities. Scientific scrutiny of such flows has been slow in coming.
For a long time, many of the scientists who studied the movement of fresh water over and through the ground felt that their purview ended at the shoreline. Meanwhile, many oceanographers knew that fresh water seeped from land into the oceans, but, suspecting that volumes were small, they considered that influx to be of little consequence. Agencies that sponsor research were often mystified about how to classify proposed projects to study the phenomenon. That confusion tended to stifle the flow of research funds, says William C. Burnett of Florida State University in Tallahassee.
Calculating the amount of water that rivers and other surface runoff carry to the sea is relatively easy. But the low flow rates characteristic of submarine groundwater discharge make it tricky to assess, and its underwater milieu makes detailed study difficult. Also, variations in porosity in the rocks carrying the discharge can cause rates of seepage to vary significantly from one spot to another, requiring scientists to take measurements over broad areas to get a good estimate of the overall flow rate. Few of the world’s coastlines have had wide-ranging surveys of their submarine groundwater discharge. Nevertheless, individual research projects, such as a study recently conducted in Florida’s Biscayne Bay, suggest that such seepage can be significant.
Water samples taken from several research wells on the Biscayne Bay floor between August 2002 and March 2004 were much less salty than water in the bay itself. This indicates that fresh water was making its way into the bay from land, says chemical oceanographer Peter W. Swarzenski of the U.S. Geological Survey in St. Petersburg, Fla.
Using direct measurements of seepage during March 2004, Swarzenski and his colleagues estimated that each day about 230 liters of fresh water enter the bay through each square meter of the discharge zone. Therefore, the volume of fresh water supplied to the bay by submarine groundwater discharge is about 6 percent of the volume entering the bay through rivers or surface runoff. The researchers reported their findings in May at a meeting of the American Geophysical Union in New Orleans.
While many stretches of the East Coast receive comparable percentages of their fresh water from submarine groundwater discharge, locales along the West Coast may receive only 1 percent, if that, says Swarzenski. But in other areas, such as along the shores of Mexico’s Yucatán, as much as 15 percent of the ocean’s freshwater input comes from submarine groundwater discharge.
Overall, data from the smattering of studies conducted over the past decade or so suggest that between 5 and 6 percent of the fresh water that makes its way to the world’s oceans does so along subterranean routes, says Burnett.
Wherever submarine groundwater discharge is occurring, the seeping water carries a variety of dissolved substances, some of which can have profound effects on local ecosystems. For example, the groundwater coming from land may contain concentrations of dissolved nitrate at least 10 times the concentrations typically found in coastal waters, says Ivan Valiela, a marine biologist at Boston University. Nitrate, which comes from septic systems, fertilizer, and decomposing marsh plants, is an important nutrient for algae and other phytoplankton at the base of the sea’s food chain.
Nutrient-stimulated algal blooms are a double-edged sword. In the short term, they provide an increased food supply for fish, but over the long haul, their decomposition can rob the water of dissolved oxygen and threaten marine life such as coral colonies.
Change of season
Just as a region’s volume of precipitation varies from season to season and from year to year, so, too, does the seepage rate of submarine groundwater. For example, ocean tides influence the rate at which groundwater seeps upward through the seafloor. During high tide, discharge sites lie beneath a tall column of water, whose pressure counteracts the fresh water’s seepage. The shorter column of overlying seawater during low tide permits more groundwater discharge.
Another factor that controls submarine groundwater discharge is the elevation of the water table on land. The higher that water table, the more pressure on the water in aquifers and the more readily water is driven offshore. Although the water table typically doesn’t fluctuate much day to day, over the course of a year, it can move up and down several meters, says Charles F. Harvey of the Massachusetts Institute of Technology.
The effects of these seasonal changes showed up during a study that Harvey and his colleagues conducted at Waquoit Bay, Mass. Data taken between 1999 and 2003 during summer months, when the water table on land was high, showed large volumes of groundwater seeping into the bay—on average, about 200 liters per hour for each square meter of discharge zone. However, data gathered in February 2004—a time of year when the water table is typically low—indicated that salt water was actually being drawn into the bay floor.
Several factors contribute to this seepage seesaw, the researchers propose in the Aug. 25 Nature. First, the aquifer recharges between late autumn and early spring. Because it takes a few months for water to filter down through the uppermost layers of unsaturated soil, the peak seepage of water into the bay doesn’t occur until summer.
From late spring until early autumn, the water table drops because growing plants are extracting more water from the soil than is returning as precipitation. So, after a few months, bay water is drawn into the submerged sections of the aquifer.
Searching for water
Increasingly, scientists are employing a variety of high-tech dowsing devices to identify undersea sites where fresh water seeps into coastal shallows.
One such instrument is a radon detector. Radon is produced by the decay of radium, a radioactive element found in nearly all soil and rock. Therefore, groundwater contains much higher concentrations of the dissolved gas than seawater does.
Because radon itself is radioactive, it makes an ideal marker for seafloor seepage, says John F. Bratton of the U.S. Geological Survey in Woods Hole, Mass. He and his colleagues developed a system that, in a matter of minutes, pumps seawater into a chamber, extracts the dissolved radon, and measures the water’s temperature and salinity. Instruments record those data along with navigational information that enables scientists to map where groundwater is seeping into coastal waters.
Another technology to identify seepage sites works somewhat as ground-penetrating radar does. In this method, scientists tow behind a boat a device that repeatedly emits and receives electrical charges to measure the electrical resistance—and hence the salinity—of the water along the ocean bottom.
The discharge sites that Burnett and his colleagues identified during research cruises in Florida’s Sarasota Bay matched those identified by radon and other geochemical tracers, he noted last December at a meeting of the American Geophysical Union in San Francisco. This electrical method is far quicker than laboratory analysis using tracers, which may take weeks to complete.
Burnett’s team also measured water temperatures and found that the waters near discharge sites in summer months were cooler than they were in the rest of the bay. Such temperature differences can easily be exploited to find discharge sites, Tomochika Tokunaga and his colleagues from the University of Tokyo said at the San Francisco meeting.
In a nighttime aerial survey of a shallow coastal site near Shiranui, Japan, these scientists used thermal infrared-imaging equipment to scan the sea’s surface over a known discharge zone. In August, the ocean temperature was about 14°C warmer than that of the groundwater seeping from the seafloor. When the seafloor spring lay 1.1 m below the water’s surface, the surface temperature was about 0.4°C cooler than that of nearby waters not situated over discharge zones.
Once seepage sites are located, scientists deploy instruments to measure flow rates. The seepage meters used most often by researchers today are 55-gallon drums that have been sawed in half and had their open ends shoved into the seafloor. Water flowing out of the sediment forces water trapped in the enclosure through a nozzle on the upper end of the drum and into a plastic bag, which can be recovered and weighed to gauge the rate of discharge—an effective but decidedly low-tech method.
Increasingly, researchers are turning to fancier gadgets to measure flow rates. In one such instrument, a computer-controlled heating coil zaps water as it enters one end of a tube placed in the seep. Sensors then measure the time it takes for the heated water to reach the other end. The speed of water flow through the tube enables scientists to calculate the rate of discharge.
Other researchers are developing seafloor instruments that can identify substances dissolved in seafloor seepage. For example, the prototype equipment designed by Arnaud Bossyut and Gary M. McMurtry of the University of Hawaii at Honolulu measures dissolved gases such as methane, carbon dioxide, and hydrogen sulfide. The device is battery powered and designed to operate at depths of up to 1 km for 6 months at a time. Future versions of the apparatus may identify traces of dissolved nitrates or other nutrients, McMurtry notes.
The recent flurry of scientific interest in submarine groundwater discharge heartens Burnett, who, with a few crusading colleagues, has apparently convinced other researchers that seafloor seepage is important. “It’s about time,” he notes.