The iron record of Earth’s oxygen
Scientists are decoding the geological secrets of banded iron formations
Iron is a gift from above.


Its atoms were forged by nuclear reactions inside massive stars that exploded, seeding our galactic neighborhood with the raw materials for planets over billions of years before the solar system formed.
Although iron is, by weight, the most abundant element in the solid Earth, most lies hidden in the planet’s core, which may be one immense, silicon-tainted iron crystal (SN: 1/12/02, p. 22). Less than 6 percent of Earth’s crust is iron, but fortunately for the voracious appetite of Industrial Man, the element is plentiful in oxide-rich ore deposits, including banded iron formations.
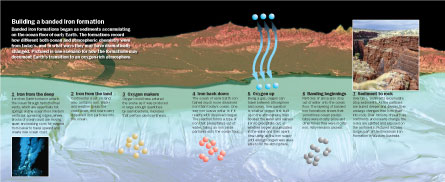
BIFs, as they’re known to geologists, are enigmatic. All seem to have started out as sediments on ancient seafloors, and by some estimates the oxide mineral accumulated in all known BIFs contains about 20 times as much oxygen as today’s atmosphere does. Yet some of these deposits accumulated long before Earth’s atmosphere became thoroughly oxygenated, so the source of the oxygen stored in these BIFs is baffling.
Then there’s the mysterious banding, in which thin layers of iron-rich minerals such as hematite (Fe2O3) and magnetite (Fe3O4) alternate with silica-rich, iron-poor bands, usually of jasper and chert.
Finally, some of the BIFs are puzzling simply because they’re so big: They stretch for hundreds of kilometers, a distance over which the banding apparently remains intact. So whatever processes created these formations must have acted over broad areas.
Countless details regarding these formations’ origins are hidden because many older BIFs are metamorphic rocks — they’ve been physically warped and chemically cooked deep within the planet, sometimes for millions of years.
“Despite the fact that people have been looking at these formations for a century or more, we still don’t have a really firm handle on where they formed in the oceans, how they formed and what they’re telling us about the composition of the oceans or the atmosphere at that time,” says geobiologist Kurt Konhauser of the University of Alberta in Edmonton, Canada. “These are, surprisingly, still the questions that are out there.”
New research is shedding some light on when, and how, some of Earth’s most voluminous banded iron formations developed. Most of the largest formations date from the late Archean eon, which ended around 2.5 billion years ago, and the early part of the eon following it, the Proterozoic. This transition between eons is thought to mark huge environmental changes, specifically the switch from a mostly methane or carbon dioxide atmosphere to an oxygen-rich one friendly to complex life. The actual timing and suddenness of the change — an occasion so momentous that scientists have dubbed it the Great Oxidation Event — generate debate.
How BIFs, which formed in ancient oceans, document environmental transitions also remains debated. One recent paper suggests that atmospheric oxygen couldn’t increase and thus couldn’t set the stage for the proliferation of multicellular life until ocean chemistry changed in a way that large BIFs stopped forming.
Other studies hint at possible reasons for BIF banding. Also, tantalizing data gathered at an unusual lake in Indonesia suggest that this body of water may be one of the few modern environments that resembles Earth’s Archean oceans.
The stage for complex life
Water can hold a lot of dissolved ions of iron — typically those that have lost two electrons (Fe2+) — but only if the water contains no dissolved oxygen. Whenever dissolved iron and dissolved oxygen get together they combine to form a nearly insoluble precipitate, which drops out of solution. In the ancient ocean, this precipitate would have accumulated as seafloor sediments.
In the late Archean, Earth’s oceans were chock-full of dissolved iron, which had either eroded from continental rocks or been spewed, along with many other dissolved minerals, into the oceans by hydrothermal activity at vents and mid-ocean ridges. Once the seas got their first whiff of oxygen produced by ocean-dwelling cyanobacteria, among Earth’s early photosynthesizers, the iron oxides that formed began to accumulate in ocean basins, says Alan J. Kaufman, a geologist at the University of Maryland in College Park.
An early idea about oxygen and BIFs is that, once the atmosphere became oxygenated, excess oxygen from the atmosphere dissolved into the ocean and caused iron to precipitate. But Kaufman suggests that, “until the dissolved iron was used up, oxygen couldn’t build up in the atmosphere.” The oxygen in the ocean couldn’t escape because iron was reacting with it, creating the sediments that would become large BIFs. Eventually, the increased supply of oxygen overwhelmed any iron that still did enter the ocean.
So, for millions of years, thin layers of iron-rich minerals rained down upon the seafloor, says Kaufman. In some places the strata — layers that today are high-quality ores containing as much as 55 percent iron by weight — stacked up hundreds of meters thick.
Analyses of African rocks by Kaufman and his colleagues thus suggest a slightly later date for oxygenation than some previous studies have: about 2.316 billion years ago, the researchers report in the May Geology. The new date is based on 58 samples of rocks that had been laid down as shallow marine sediments in what is now north central and northeastern South Africa between 2.65 billion and 2.1 billion years ago. In the oldest samples, the relative proportions of four sulfur isotopes, particularly the ratios involving sulfur-33 and sulfur-36, suggest that sulfur-bearing compounds were often involved in chemical reactions driven by ultraviolet light. Then, in rocks deposited late in that interval, sulfur ratios shifted to those that typically result from metabolic reactions within organisms.
First reported in 2001 by James Farquhar, a coauthor on the Geology paper who is also at the University of Maryland in College Park, and others, the changes in the ratios of sulfur isotopes are a key tool for pinpointing when the rock record documents the shift to a more oxygenated atmosphere.
Interruption of the UV-driven reactions at 2.316 billion years ago marked an important milestone in Earth’s history: the formation of an ozone layer, Kaufman and colleagues report. That UV-blocking layer would have begun to form when atmospheric concentrations of oxygen rose to about 1/100,000th of modern-day levels, says Kaufman.
The appearance of the ozone layer triggered a series of biological and environmental changes that set the stage not only for life to advance from one-celled to multicellular forms, but also for the world’s first ice age, the researchers contend. First, Kaufman explains, any UV-sensitive organisms living in deep waters would have been able to rise to shallower depths once the ozone layer had developed. There, the photosynthesizing organisms could have taken advantage of increased light, boosting their populations and their oxygen production dramatically. In probably less than 10 million years, oxygen levels increased to about 1 percent of today’s value.
As oxygen was building in the atmosphere, it reacted with methane, then a major atmospheric constituent, to produce carbon dioxide. Carbon dioxide is a planet-warming greenhouse gas, but methane warms the air about 62 times as well as carbon dioxide does, says Kaufman. An outcome of the atmospheric change was a cooling effect so strong that it sent the world into an ice age, he says.
Additional oxygen in the air would also have increased the rates of weathering and erosion on land. Combined with the scouring of rocks by glaciers, these effects would have sent more nutrients off the continents and into the seas, boosting even further the populations of oxygen-producing marine organisms.
Finally, Kaufman notes, atmospheric oxygen might have driven the evolution of organisms such as eukaryotes that use, rather than make, oxygen for several important biochemical pathways.
Another way ocean chemistry could have set the scene for oxygen accumulation was by a decrease in the production of methane (SN: 5/9/09, p. 14), which reacts with oxygen. Other researchers suggest that ratios of nickel to iron in banded iron formations document that, starting about 2.7 billion years ago, geological changes began to reduce the amount of nickel spewing from volcanoes or eroding into the sea. The populations of microbes that used the nickel to produce methane dropped. With less methane in the atmosphere, oxygen emitted by cyanobacteria could eventually build up, Konhauser and his colleagues report in the April 9 Nature.
Seas of old
Although most banded iron formations probably accumulated during or after cyanobacteria began suffusing oxygen throughout the seas, some of the formations — albeit ones substantially smaller than those that formed about 2.5 billion years ago —formed millions of years before any dissolved oxygen may have been available.
Scientists have long debated how in such a world dissolved iron became oxidized sans oxygen, losing an electron and going from Fe2+ to Fe3+. Something other than oxygen must have acted as an electron acceptor, says Sean A. Crowe, a biogeochemist at the University of Southern Denmark in Odense. Not only was free oxygen in short supply, oceans then contained little if any dissolved sulfate, an electron acceptor produced when sulfur dioxide spewed by volcanoes reacts with oxygen and water.
Lab tests show that ultraviolet light can stimulate the oxidation of dissolved iron in anoxic water, which contains no dissolved oxygen. But no one has observed such a reaction in anoxic seawater or its laboratory equivalent, Crowe says. The only other known process for oxidizing dissolved iron in anoxic water involves ancient microbes known as photoferrotrophs, organisms that derive energy from light and iron. Now, Crowe and his colleagues have discovered an Indonesian lake where photoferrotrophs thrive. The deep portion of the lake could be a modern-day version of the Archean ocean.
Lake Matano in Indonesia is only 28 kilometers long and, at the widest point, only 8 kilometers across. But at its deepest it is more than 590 meters deep. Its great depth and steep-sided bottom, along with the region’s lack of strong seasonal temperature variations, contribute to poor circulation in the lake, the researchers reported last October in Proceedings of the National Academy of Sciences.
Field measurements reveal that Lake Matano’s surface waters are oxygenated but that all waters below depths of 100 meters, which account for most of the lake’s volume, are anoxic. Because soils in the surrounding watershed are poor, few nutrients make their way into the lake, says Crowe. The few dissolved sulfates that wash in are consumed by chemical reactions that occur within the chemocline, the thin layer of water that separates the oxygenated surface waters from the anoxic depths. So, the researchers note, the oxygen- and sulfate-free depths of the lake, which also happen to be rich in dissolved iron, probably are chemically similar to Earth’s oceans as they existed before the atmosphere became thoroughly oxygenated.
Few photosynthetic microbes live in the nutrient-poor surface waters of the lake. Those that do, like most plants and marine organisms that thrive in oxygenated conditions, use a type of chlorophyll called chlorophyll a. The dearth of microbes renders the lake’s upper waters exceptionally clear, which allows some sunlight to penetrate anoxic waters. At a depth of about 120 meters, Crowe and his colleagues found that the most common microbes lacked chlorophyll a but contained bacteriochlorophyll e, a light-harvesting pigment that absorbs infrared light and is present in photoferrotrophs adapted to low-light conditions. Genetic analyses suggest that photoferrotrophs are among Earth’s earliest microbes.
These microbes live at a depth where light levels are between 0.005 and 0.01 percent what they are at the lake’s surface, says Crowe. Yet the organisms thrive: Each liter of water there contains between 300 million and 16 billion of the photoferrotrophs, the researchers estimate. Those microbes live in the anoxic sweet spot where dissolved iron and phosphorus rising from the depths meet the weak-yet-usable sunlight filtering down from above. Their rate of growth creates just as much biomass —about 650 nanograms of carbon per hour in each liter of water —as photosynthetic organisms at the lake’s surface do.
“This is a first glimpse at a microbial ecosystem in a stable, iron-rich aquatic environment,” says Crowe. “This really makes the argument for a biological role in the formation of banded iron formations much more believable.” The team estimates that photoferrotrophs could have, on a worldwide basis, produced about 10 percent of the biomass that modern photosynthetic organisms do.
Crowe and his colleagues didn’t actually discern Lake Matano’s microbes converting dissolved iron to its insoluble form: In those light-limited conditions, it would take months to produce measurable quantities, he notes. In the future, lab tests on microbes cultured from those taken from Lake Matano’s depths may yield further insights about how ancient microbes might have thrived in Archean oceans, and what sort of nutrients or trace elements they would have needed to fuel their metabolisms.
Not-so-temperate banding
One of the biggest mysteries about banded iron formations is what caused the alternating layers of iron-rich and silica-rich strata, which can range anywhere from a few micrometers to a few meters thick. While some research hints that the thickest bands may be associated with long-term climate changes that stem from variations in Earth’s orbit, what triggers micrometer layering has been more puzzling, says University of Alberta’s Konhauser.
Previously, some teams have proposed that the small-scale layering derives from regular variations in the supply of iron-rich anoxic waters from the ocean depths — due to recurring pulses of hydrothermal activity or from climate-driven changes in ocean currents, Konhauser says. But now, lab tests by him and his colleagues hint that something much simpler — seasonal changes in ocean temperature — may have caused the banding. The team reported their findings last September in Nature Geoscience.
The team measured the rates at which several photoferrotroph types oxidized dissolved iron at various temperatures in anoxic, silica-rich water samples. For all of the microbes tested, oxidation rates increased as water temperature rose from 5° Celsius to 25°C. At temperatures of 30°C and higher, oxidation rates dropped substantially. When the water temperature rose to 55°C, the photoferrotrophs continued to oxidize iron but were eventually incapacitated: Even after temperature returned to 25°C, the microbes had stopped oxidizing iron and didn’t restart. Similarly, the microbes probably wouldn’t survive prolonged exposure to waters spewing from hydrothermal vents or volcanic seamounts.
While iron oxidation caused iron-rich minerals to drop out of solution as water temperatures rose from 5°C to 25°C, the precipitation of silica showed exactly the opposite trend, Konhauser says. At high temperatures, silica remained dissolved; as waters cooled, silica crystallized out of solution. These disparate trends could explain the banding seen in BIFs, the researchers propose: During ancient summers, photoferrotrophs proliferated and oxidized iron prodigiously, sending a cascade of iron-rich minerals to the seafloor. In winter, iron stayed in solution because the microbes weren’t active, but cool temperatures caused silica-rich minerals to precipitate.
“Despite these experiments, the mechanisms of BIF deposition are still an area of great uncertainty,” says Konhauser. Looking at the ratio of iron isotopes in minerals precipitated by photoferrotrophs in lab tests, and comparing the ratios with those in BIFs, may provide insights into whether similar reactions occurred before the formation of an ozone layer. Also, he notes, the results of such experiments may illuminate which trace elements are metabolically required by modern-day photoferrotrophs, and comparisons with the elements present in ancient BIFs could reveal which types of microbes were most likely the progenitors of today’s iron ore deposits.