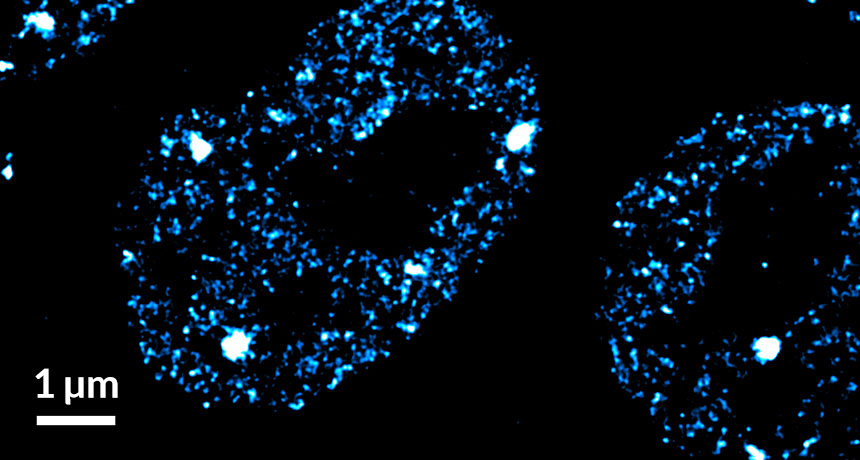
MARKING THE SPOTS In this super-resolution microscope image of mouse embryonic stem cells, molecules of the enzyme that copies DNA instructions into RNA messages cluster in multiple spots within the nucleus. White spots have more of the enzyme, RNA polymerase II, concentrated in them than in the cooler blue spots.
W.-K. Cho et al/Science 2018
Turning on genes may work like forming a flash mob.
Inside a cell’s nucleus, fast-moving groups of floppy proteins crowd together around gene control switches and coalesce into droplets to turn on genes, Ibrahim Cissé of MIT and colleagues report June 21 in two papers in Science.
Researchers have previously demonstrated that proteins form such droplets in the cytoplasm, the cell’s jellylike guts. Some, including Cissé’s MIT colleagues Richard Young and Phillip Sharp, have proposed that this process — called phase separation — could also happen in the nucleus when cellular machinery turns genes on, which involves copying DNA instructions into RNA messages.
If confirmed, the discovery challenges earlier ideas that gene activity is controlled by single molecules of stable protein complexes that remain stuck to DNA for long periods.
Cissé and colleagues used super-resolution microscopy to view single molecules of protein in live mouse embryonic stem cells. In particular, they were interested in RNA polymerase II, an enzyme that copies DNA into RNA, and parts of the Mediator complex, a group of proteins that help kick-start that copying process, called transcription. The researchers tagged the proteins with a fluorescent protein and watched what happened.
RNA polymerases II and Mediator proteins each formed large clusters, each with about 200 to 400 molecules. Those clusters had properties of phase-separated droplets: Each cluster formed distinct dots when viewed through the microscope. Those dots could fuse together, like oil droplets merging in water. And the droplets could be dispersed with alcohol. That’s convincing evidence that Cissé sees phase-separated condensates, says Anthony Hyman, a biologist at the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, Germany. He was not involved in the work.
While these clusters seem stable, the researchers found that individual polymerase or Mediator proteins were constantly darting in or out of the cluster. About 90 percent of polymerase molecules and 60 percent of Mediator molecules spent onlyabout 10 seconds in the clusters, the team found.
That’s a sharp contrast to previous studies suggesting that RNA polymerase II stays at a gene for minutes to hours, says single-cell biochemist Robert Tjian, a Howard Hughes Medical Institute investigator at the University of California, Berkeley. “The biggest surprise is how fast these things are happening,” Tjian says. He and colleagues also found that these proteins interact only briefly, most for just five to 20 seconds.
These protein mobs are drawn together by their floppy bits, called low complexity or intrinsically disordered regions, Tjian and colleagues report June 21 in Science. Floppy, intrinsically disordered proteins are needed for a wide variety of cellular processes (SN: 2/9/13, p. 26).
In Cissé’s studies, Mediator proteins clustered with groups of gene control switches called super-enhancers. Typically, super-enhancers are located far from the genes they regulate, and may control several genes, sometimes simultaneously. Droplets containing Mediator proteins and the super-enhancers may interact with RNA polymerase II-containing droplets to spur transcription, the findings suggest.
Sudden coalescence of proteins into droplets could help explain why genes turn on in a flash, Hyman says. Bubbles of enhancers might interact with multiple bubbles of RNA polymerase II to turn on several genes at once, something that was hard to explain if single protein molecules were responsible.
Tjian doesn’t call what he sees phase separation, even though his results are similar to Cissé’s. It’s not necessary to concentrate proteins so densely that they will form droplets in order to get transcription started, he says. Instead, proteins clustered in hubs can spur transcription at a range of concentrations, he found.
But Julie Forman-Kay, a biophysical chemist at the Hospital for Sick Children in Toronto, says Tjian is making a semantic argument. “What he calls a hub is, in my book, evidence for phase separation,” she says. All of the studies show the same thing, she says: Clusters of floppy proteins concentrate in particular locations to turn on genes.
But the association between phase separation and transcription hasn’t been proven yet, says biochemist Danny Reinberg of New York University Langone Health. He agrees with Tjian’s findings that weak interactions between proteins can add up to a strong push to do something, in this case to copy DNA into RNA. But he needs more evidence that transcription is spurred by phase separation into droplets, as Cissé and colleagues describe. “I’m not saying it’s not happening,” Reinberg says. “I’m saying it can happen, it might be happening, but there’s no proof of that in these two papers.”







