Microbial Materials
Scientists co-opt viruses, bacteria, and fungi to build new structures
Bone. Nerve. Muscle. Horn. Hide. Silk. With ingenious assemblages of atoms and molecules, biology produces fantastic substances that have long inspired scientists to develop the synthetic materials of the modern landscape. Lately, materials scientists have turned to biology’s smallest individuals–viruses, bacteria, and fungi. Not only can these microbes be coaxed to produce high-tech components, but they can also themselves serve as valuable ingredients in new classes of materials.
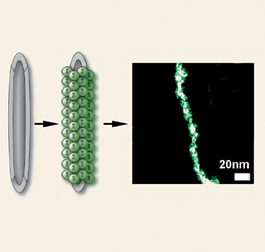
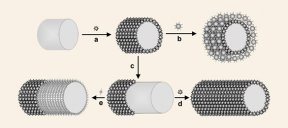
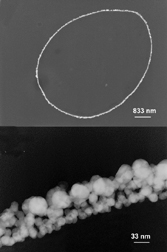

Scientists are beginning to employ microbes, for example, to organize crystals into complicated geometries or provide living templates for growing crystals. Since the structure of materials is intimately linked to their behavior, a new means of controlling crystal organization creates a buzz among materials scientists.
Microbes have several advantages as laboratory reagents. Some microorganisms, such as viruses, measure tens of nanometers in length. Researchers can’t make uniform synthetic particles at this scale, but microbes are readily available, uniform in size, and easy to work with. Because they typically live under comfortable conditions of temperature, pressure, and acidity, microbes are candidates for development of manufacturing techniques that are more environmentally friendly than today’s often hot, high-pressure, and caustic processes.
Microorganisms “represent tremendous untapped potential” for materials science, says chemist Chad Mirkin of Northwestern University in Evanston, Ill.
Hiring microbes
Many microbes produce inorganic substances of interest to materials scientists. Single-celled, ocean organisms known as diatoms make silica, the silicon-and-oxygen mix of typical glass (SN: 1/26/02, p. 51: Available to subscribers at Making silicon naturally: Chemists glimpse organic substance in plankton). Other microbes formulate nanoscale magnetic particles out of iron oxides.
Some microbes consume metals and then excrete them in precise configurations. One species of Pseudomonas bacteria lives in ore deposits rich in silver–a metal that’s generally toxic to microorganisms–and produces tiny, silver-laden crystals with specific shapes (SN: 12/4/99, p. 367). Two years ago, a team from the National Chemical Laboratory in Pune, India, reported that a fungus called Verticillium can be induced to fabricate silver nanoparticles within its cells when it’s placed in a silver nitrate solution.
Bacteria and other microbes can be genetically engineered to interact with the material world in unusual ways. Three years ago, for example, biologist Stanley Brown of the University of Copenhagen and his colleagues used bacteria to create crystals of gold. They examined millions of Escherichia coli bacteria that were genetically engineered to sport different proteins on their surfaces. The scientists sifted through this bacterial library to isolate the microbes that bind to gold particles.
The researchers then found that three of the gold-binding proteins that they’d detected on E. coli, when used in isolation from the bacteria, sped up the formation of gold crystals in a solution containing dissolved gold. This accelerated growth influenced the resulting crystals’ shapes.
More recently, in the Dec. 17, 2002 Advanced Materials, Brown and his coworkers reported that they had isolated and characterized proteins from other genetically engineered E. coli that can distinguish between very similar crystal faces of zeolites–porous inorganic crystals that are used to separate molecules and catalyze chemical reactions. The faces of a zeolite crystal, which is made of aluminum and silicon, have the same atomic makeup but subtly different structures. Ultimately, chemists might specify which surface of a crystal provides the substrate for growing another material.
Brown and his colleagues now are using libraries of genetically engineered E. coli to find proteins that bind other inorganic materials, such as mica. A geneticist, Brown is interested in how a microbe’s genes produce proteins that interact with different inorganic surfaces. He notes that materials scientists and chemists may find this information useful for engineering new structures. In time, these bacterial proteins might become another material-making tool in the inorganic chemist’s toolbox, says Brown.
Virus lineup
Viruses offer materials scientists still more possibilities. No one has been able to uniformly synthesize rod-shaped polymers the size of viruses, says physicist Seth Fraden of Brandeis University in Waltham, Mass. Yet scientists are especially interested in particles of that size because they organize themselves into structures resembling liquid crystals and so could open new routes for controlling synthesis of materials.
When the virus particles arrange themselves this way, they can move more freely in a solution (SN: 8/15/98, p. 108). If viruses instead cluster randomly, Fraden says, they’ll bump into each other and jam up like logs on a river.
To investigate how virus-size particles organize themselves, Fraden and his colleagues are now genetically engineering viruses to have precise lengths, mixing them with polymer spheres, and then examining the structures that spontaneously form.
Materials scientists are already exploiting such self-assembly strategies for controlling–down to the nanometer scale–the structures of new materials that they design.
For example, Angela Belcher of the Massachusetts Institute of Technology now employs viruses coated with various inorganic materials. The adorned viruses assemble into intricate structures that are potentially useful for building a new generation of optical, magnetic, and electronic devices.
During her doctoral work at the University of California, Santa Barbara, Belcher studied how natural materials, such as abalone shells, grow. Later, Belcher says, she decided to “move on to other materials that nature hasn’t worked with yet.”
In the May 3, 2002 Science, Belcher describes how she genetically altered the proteins at the tips of bacteria-infecting viruses, known as bacteriophages, so that they bound to zinc sulfide semiconductor crystals called quantum dots. These viruses weren’t just a curiosity. When Belcher placed them in a solution at sufficiently high concentrations, they organized themselves into a liquid-crystal-like structure in which the quantum dots were aligned. Such control of quantum dot-containing material is otherwise difficult to attain, she says.
In more recent work, reported in the May 2 Advanced Materials, Belcher’s group demonstrates a method to attach viruses to a wide variety of organic and inorganic substances, including gold nanoparticles and fluorescent dye molecules. The viruses with their attachments then assemble into fluid, yet well-organized, two- or three-dimensional structures.
Belcher and her coworkers from MIT and the University of Texas at Austin recently developed a strategy for making viruses into miniature wires that they describe in the June 10 Proceedings of the National Academy of Sciences. The team genetically altered viruses so that the protein coat along the length of each microbe was covered with peptides that bound either zinc sulfide or cadmium sulfide.
Researchers in her lab are engineering viruses to have specific chemical groups at each end of the virus as well as pick up a coating of semiconductor along its length. The team expects that those chemical groups will serve as specialized connectors that the researchers can use to link the semiconductor-coated viruses into specific combinations and structures on a surface. In effect, this would create semiconductor wires that can automatically latch together. In time, Belcher says, her team would like to arrange such wires to create simple electronic devices that are far smaller than those in conventional electronic chips.
There are also alternative uses for viruses with specific chemical groups assigned to each end. Belcher suggests that one end of a virus might be designed to carry a magnetic material, and the other a chemical group that binds to a toxic pollutant. Theoretically, researchers could then use such designer particles and a magnet to sponge up pollutants from a solution.
Protein wizardry
Mark Young of Montana State University and his colleagues are focusing on another aspect of viruses. The scientists can modify viruses’ protein shells both chemically and genetically. They custom-design these cages to bind particular materials and adjust the way the cages open and close to let particles in and out.
In effect, the cages provide nanoscale hands for assembling new materials, tiny piece by tiny piece. By placing magnetic crystals such as maghemite or magnetite inside the cages, Young and his collaborators aim to create magnetic data storage devices.
The researchers have also isolated and modified cages of protein from bacteria and archaea that resemble iron-storing ferritin cages in mammalian cells. The scientists aim to intersperse virus cages with the ferritin-like cages to create two-dimensional arrays that could be incorporated into magnetic data-storage devices.
While microbes’ benign living conditions might prove a boon to environmentally friendly manufacturing, they may limit the organisms’ use in the harsh environments of many current production processes. Young and his coworkers have taken aim at these restrictions in two ways.
They collect so-called thermophile microorganisms from the hot springs at Yellowstone National Park and also chemically modify the protein coats of conventional viruses to withstand variations in temperature and acidity.
So far, Young and his collaborators have identified or crafted protein cages that handle a pH range from 0 (extraordinarily acidic) to 11 (somewhat basic). Some of these protein cages can survive temperatures above 100C, Young says. These advances promise to extend the potential marriage of microbes and materials synthesis into new, even more technologically challenging territories.
Because the structures of the protein shells of many viruses are well understood even at atomic scales they can be particularly useful as nanoscale building tools, says M.G. Finn of the Scripps Research Institute in La Jolla, Calif. Last year, Finn and his colleagues reported genetically engineering cowpea mosaic viruses to make them bear sulfur-containing amino acids, to which the researchers subsequently bound gold particles and fluorescent dyes (SN: 2/2/02, p. 68: Available to subscribers at Viral parts: Chemists convert virus into nanoscale tool). The scientists aim to use these constructions as building blocks for electronic circuits and new materials.
Since last year, Finn’s group has expanded its repertoire to a few more viruses that the scientists collect from cells they grow in their lab. None of these viruses infect humans, Finn points out.
In yet another approach to high-tech materials, the researchers are attaching single-stranded DNA to the plant viruses, which they can then link to other materials via complementary DNA strands.
In this way, the viruses aggregate into two- and three-dimensional structures that also may prove useful for constructing electronic devices.
Glitzy fungi
According to Mirkin, fungi can provide “truly living templates” for designing materials with specific nanoscale and microscale features. In his lab, researchers have gold-plated a meshlike tangle of thin fungal fibers known as hyphae, which are very uniform in diameter and have a characteristic width for each fungus species.
In the May 23 Angewandte Chemie International Edition, Mirkin’s team at Northwestern University describes how it cultured spores of the fungus Aspergillus niger in the presence of 13-nm-wide gold particles that aggregated on the fibrous hyphae. Once there, the gold particles, each of which was linked to multiple short strands of DNA, could bind an additional nanoscale component bearing complementary DNA strands. This provides a means for readily mixing and matching a variety of nanoscale building blocks into ever more sophisticated structures, Mirkin says.
The team has already demonstrated the template procedure for additional fungi, which have different hyphae dimensions. This technique could be used to coat microbes with a variety of materials, such as magnetic and semiconductor particles, Mirkin says. He suggests that hyphae also might be custom-coated with catalytic materials to provide a large surface area for catalysis in chemical reactions. Meanwhile, other fungus-based nanostructures might serve as designer optical, electronic, and magnetic materials.
By doing nanoscale construction work for scientists, these fungi, viruses, and bacteria may make material design easier. The marriage between microbes and materials science ought to thrive.
After all, a material’s architecture at microbial scales largely determines what the substance can do.
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.
To subscribe to Science News (print), go to https://www.kable.com/pub/scnw/
subServices.asp.
To sign up for the free weekly e-LETTER from Science News, go to http://www.sciencenews.org/subscribe_form.asp.







