Misfolded proteins implicated in more brain diseases
Alzheimer’s, other disorders show similarity to Creutzfeldt-Jakob disease and other prion infections
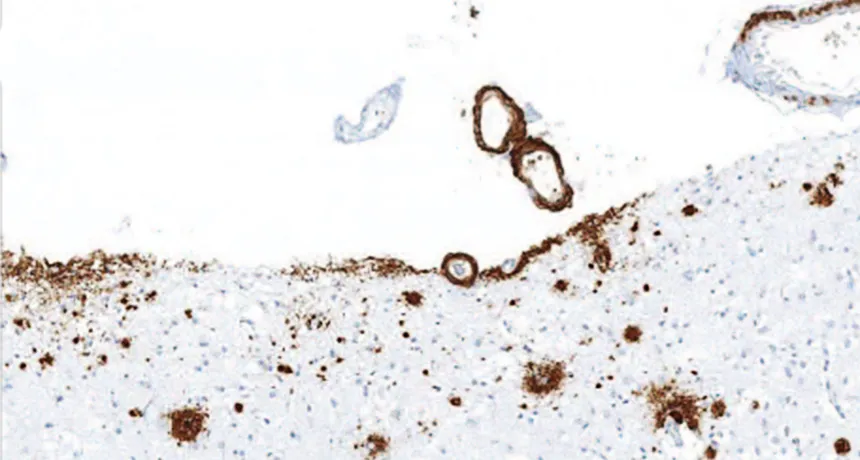
BUILDUP Amyloid-beta (brown) accumulated in the front of the brain in a person who received injections of cadaver-derived growth hormone as a child, suggesting that the injections were contaminated with A-beta.
Z. Jaunmuktane et al/Nature 2015
In some brain diseases such as Alzheimer’s, distorted proteins behave like infectious agents, spreading among brain cells and corrupting other proteins. New studies suggest that such diseases should be classified among disorders caused by the infectious particles known as prions.
Classic prion infections, such as Creutzfeldt-Jakob disease, are fatal. Some scientists hope that recasting Alzheimer’s and other neurodegenerative disorders as prion diseases may help reveal ways to halt or prevent neural destruction. Yet others caution that this radical reclassification may unnecessarily evoke fear.
Mindful of public panic, researchers are quick to say that there is no evidence that Alzheimer’s, Parkinson’s and other neurodegenerative diseases can be transmitted through normal everyday contact. “There is not one iota of evidence whatsoever that infectivity can occur from one individual to another,” says cell biologist and neuroscientist George Bloom of the University of Virginia in Charlottesville.
But scientists can’t rule out a jump from person to person under special circumstances, such as when contaminated tissue makes its way into a healthy body via certain medical procedures.
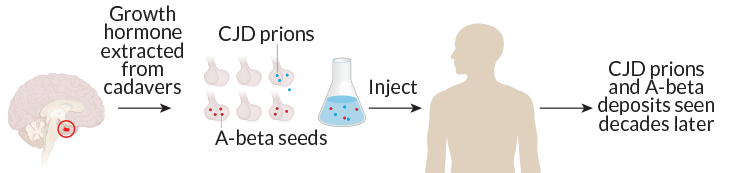
That exact scenario may have occurred in people who received injections of growth hormone derived from cadaver pituitary gland tissue, scientists report online September 9 in Nature. A postmortem study of eight such people who died between the ages of 36 and 51 found that four had substantial amyloid-beta buildup in their brains, a sign of Alzheimer’s disease.
“This was a highly unusual finding,” study coauthor John Collinge of University College London said September 8 in a media briefing. “You wouldn’t have expected to see this Alzheimer’s pathology in this age group.”
The patients had all acquired Creutzfeldt-Jakob disease from prion-contaminated injections. Finding A-beta deposits in these relatively young brains suggests that those contaminated injections may have been laced with A-beta prions, too.
Scientists can’t rule out other explanations for the presence of A-beta in these people’s brains. But the results raise the possibility that given the right opportunity, A-beta and other proteins involved in brain disorders, such as the Alzheimer’s-related tau and Parkinson’s-linked alpha-synuclein, may propagate through the brain like prions.
Thinking of the proteins involved in neurodegenerative diseases as prions “brings conceptual unity to the whole field,” says neurobiologist Michel Goedert of the University of Cambridge.
At the heart of prion diseases lie deformed proteins. Like bad apples, these contorted proteins coax normal proteins to change shape into misfolded forms. A benign shape of looping structures called alpha helices, for instance, might transform into more tightly packed shapes called beta-sheets. Through a process that remains mysterious, these bundles of beta sheets then turn dangerous.
Most of what’s known about these infectious prions — short for “proteinaceous infectious particles” — comes from the original prion protein identified by University of California, San Francisco researcher Stanley Prusiner in the 1980s. Prion proteins exist in a normal form in the body. When shape-shifted into a dangerous conformation, the protein causes diseases such as Creutzfeldt-Jakob disease and kuru, a neurodegenerative disorder acquired through ritualistic cannibalism of brain tissue among tribal New Guineans (SN: 7/11/15, p. 11).
In classic prion diseases, the infectious agents spur other proteins to misfold as they move from cell to cell and from animal to animal. A-beta seems to do something similar, recent animal studies suggest. Buildup of A-beta has been spotted in the brains of mice that received injections of tissue from mouse brains with signs of Alzheimer’s, scientists reported in 2011 in the Journal of Neuroscience. A-beta can also spread from cell to cell, Kurt Giles of UC San Francisco, Prusiner and colleagues reported in Proceedings of the National Academy of Sciences in 2012. Similar behavior has been spotted for the Alzheimer’s-related protein tau.
And like the original misfolded prion protein, A-beta seeds seem to persist for long periods. When scientists put clumps of A-beta into mice that lacked even normal A-beta, nothing happened. But when brain tissue from those mice was injected into mice that did have A-beta, the formerly inert seeds sprang into action and regained their damaging behavior, neurobiologist Mathias Jucker of the University of Tübingen in Germany and colleagues report September 9 in Nature Neuroscience.
Scientists have also observed troublesome behavior by a protein called alpha-synuclein, which aggregates in Parkinson’s disease and a related neurological disease called multiple system atrophy, or MSA. Mice genetically altered to have a faulty copy of the human alpha-synuclein protein received injections of brain tissue from 14 people who suffered from MSA. After 120 days, alpha-synuclein had accumulated in the mice’s brains and caused damage, Giles, Prusiner and colleagues reported August 31 in the Proceedings of the National Academy of Sciences.
These results could pose a problem for surgical teams, Giles and colleagues hint in the paper. Hardy prions resist standard decontaminating procedures used by hospitals, so surgical equipment that comes into contact with brain tissue may need more thorough cleaning procedures. What’s more, lab workers who study tissue from MSA or other disorders may need to be extra cautious, Giles says.
Older, less direct evidence for alpha-synuclein’s spread comes from people with Parkinson’s who received fetal stem cell transplants. When participants died, their transplanted cells were rife with alpha-synuclein, two 2008 postmortem analyses revealed. That finding suggested that the misfolded alpha-synuclein proteins had spread to the healthy young grafts. “That was the first indication that the protein can infect the surrounding cells,” says neuroscientist Jiri Safar of Case Western Reserve University, who also runs the National Prion Disease Pathology Surveillance Center in Cleveland.
So far, brain tissue from people with Parkinson’s disease doesn’t seem infectious in mouse experiments, Giles says, suggesting that the alpha-synuclein at work in MSA might be different from the one in Parkinson’s. That idea raises one of the biggest outstanding mysteries in the field: defining what constitutes an infectious “seed.”
A-beta, for instance, comes in multiple shapes and sizes. It’s possible that A-beta and other proteins that aggregate exist as large, complex mixtures called “clouds.” Scientists don’t know which form is the most likely to spread, or which form is most dangerous for cells. Those two attributes may very well rely on different forms, Goedert says.
While animal studies have proved useful in illuminating how these proteins can behave in certain situations, there’s still much to learn about how they actually work in the human brain, Goedert says. “The question is, what do they mean for human disease?”
Because the research tying these neurodegenerative diseases to prions is in its infancy, scientists are still wrangling over what to call these disorders. “Within the scientific community, everyone seems to have their own definition of what a prion disease is,” Giles says.
Some oppose expanding the definition of prion diseases. “The word ‘prion’ induces a lot of fear,” says neurologist Valerie Sim of the University of Alberta in Edmonton, Canada. And an outsized public reaction could have consequences such as denials of surgeries for people with these disorders and shuttering of research labs, she says. This expanding umbrella of prion disease is “trying to redefine a scary word,” she says.
Others, such as Goedert, prefer to say these diseases are “prionlike.” That could convey that in some ways, the diseases are similar to classic prion diseases, as in their the cell-to-cell spreading; in other ways, such as their lack of infectivity, prionlike diseases differ. “I think one should not call Alzheimer’s disease and Parkinson’s disease prion diseases at this point,” he says. “But I think it’s true to say there are similarities.”
Semantics aside, researchers agree that approaching these neurodegenerative diseases as disorders of protein folding and spreading may lead to insights on how to stop or prevent them, providing therapies that are desperately needed and have proven elusive so far.
One approach borrowed from the prion field would be to lower the number of uncorrupted proteins in the brain. Much like the reduction of dry kindling can prevent forest fires, getting rid of normal proteins might be a way to prevent their ultimate destruction.
The field is young, and evolving quickly, says Giles. “Watch this space,” he says. “I think the next few years will tell us whether or not this is a useful way to go.”