Paddle Power: Surprising shape of key cellular pore unveiled
Underlying every thought and bodily motion are nerve and muscle cells sending or receiving electrical signals. Driving this activity are voltage-gated ion channels–pores that quickly open or close to various ions depending on the electrical properties of the cell’s membrane.
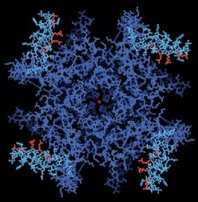
A research team has now obtained the first atomic-scale portrait of one of these crucial gatekeepers, a voltage-dependent potassium channel. “It looks very different than any of us expected,” says team leader Roderick MacKinnon, a Howard Hughes Medical Institute (HHMI) investigator at Rockefeller University in New York
Over the past 6 years, in work that some scientists predict will earn a Nobel prize, MacKinnon’s team isolated the proteins that make up various kinds of ion channels, coaxed them into forming stable crystals, and beamed X rays through the crystals to locate the positions of atoms within the pores. From such data, the researchers uncovered often-unexpected structures for channels that regulate the flow of potassium, sodium, calcium, and other ions (SN: 3/9/02, p. 152: Channel Surfing).
However, voltage-dependent ion channels resisted the team’s attempts at crystallization. MacKinnon and his colleagues finally circumvented that obstacle by binding an antibody to each of the four subunits that make up a voltage-dependent potassium channel. This stabilized the structure enough to form crystals that the investigators could analyze.
Most scientists had thought that the voltage-sensing regions of the pore would be hidden within the ion channel, but instead they jut out as hinged projections that MacKinnon calls “paddles.” In hindsight, he adds, it’s clear that the free-moving nature of these four, positively charged paddles accounts, at least in part, for the difficulties in crystallizing the pores.
Taking into account the new structure and several follow-up experiments, all described in the May 1 Nature, MacKinnon’s team has offered a proposal for how voltage-gated ion channels work. When the inner portion of a cell membrane has a negative charge, the paddles of the pore are attracted to it and extend horizontally along that inner membrane, keeping the channel closed. If the outer half of the cell membrane becomes negatively charged, however, the paddles assume a vertical position by swinging on their hinges toward that charge. “That moving up pulls the pore open,” suggests MacKinnon.
“The structure is a complete surprise to the field,” says David Clapham, an HHMI investigator at Children’s Hospital in Boston. Most ion-channel researchers had never envisioned such large movements by the voltage-sensing parts of the pores. “I am awed by the high quality of the work and the courage of the interpretation,” says Clapham.
The potassium channel that MacKinnon’s team probed actually comes from a bacterium, so scientists don’t yet know whether its structure reflects a general shape for all voltage-gated ion channels, notes Clapham, who studies a voltage-sensitive sodium channel.
Because the amino acid makeup of the bacterial potassium channel is similar to that of other voltage-gated channels, especially in the paddle regions, it’s likely that all such channels operate in the same way, predicts MacKinnon.
The discovery that the voltage sensors of the ion channel are more exposed than scientists had expected may interest those looking for drugs that influence nerve or muscle activity. “I’m thinking now very differently about potential targets for drugs,” says MacKinnon. “I think these paddles are very promising targets.”
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.