A gut-brain link for Parkinson’s gets a closer look
The misfolded proteins may start with microbes in the digestive system

GUT INSTINCT John Carlin (left) was diagnosed with Parkinson’s disease 16 years ago. His wife, Martha (right), founded a company to help research the theory that gut microbes play a role in the disease.
Matt Nager
Martha Carlin married the love of her life in 1995. She and John Carlin had dated briefly in college in Kentucky, then lost touch until a chance meeting years later at a Dallas pub. They wed soon after and had two children. John worked as an entrepreneur and stay-at-home dad. In his free time, he ran marathons.
Almost eight years into their marriage, the pinky finger on John’s right hand began to quiver. So did his tongue. Most disturbing for Martha was how he looked at her. For as long as she’d known him, he’d had a joy in his eyes. But then, she says, he had a stony stare, “like he was looking through me.” In November 2002, a doctor diagnosed John with Parkinson’s disease. He was 44 years old.
Carlin made it her mission to understand how her seemingly fit husband had developed such a debilitating disease. “The minute we got home from the neurologist, I was on the internet looking for answers,” she recalls. She began consuming all of the medical literature she could find.
With her training in accounting and corporate consulting, Carlin was used to thinking about how the many parts of large companies came together as a whole. That kind of wide-angle perspective made her skeptical that Parkinson’s, which affects half a million people in the United States, was just a malfunction in the brain.
“I had an initial hunch that food and food quality was part of the issue,” she says. If something in the environment triggered Parkinson’s, as some theories suggest, it made sense to her that the disease would involve the digestive system. Every time we eat and drink, our insides encounter the outside world.
John’s disease progressed slowly and Carlin kept up her research. In 2015, she found a paper titled, “Gut microbiota are related to Parkinson’s disease and clinical phenotype.” The study, by neurologist Filip Scheperjans of the University of Helsinki, asked two simple questions: Are the microorganisms that populate the guts of Parkinson’s patients different than those of healthy people? And if so, does that difference correlate with the stooped posture and difficulty walking that people with the disorder experience? Scheperjans’ answer to both questions was yes.
Carlin had picked up on a thread from one of the newest areas of Parkinson’s research: the relationship between Parkinson’s and the gut. Other than a small fraction of cases that are inherited, the cause of Parkinson’s disease is unknown. What is known is that something kills certain nerve cells, or neurons, in the brain. Abnormally misfolded and clumped proteins are the prime suspect. Some theories suggest a possible role for head trauma or exposure to heavy metals, pesticides or air pollution.
People with Parkinson’s often have digestive issues, such as constipation, long before the disease appears. Since the early 2000s, scientists have been gathering evidence that the malformed proteins in the brains of Parkinson’s patients might actually first appear in the gut or nose (people with Parkinson’s also commonly lose their sense of smell).
From there, the theory goes, these proteins work their way into the nervous system. Scientists don’t know exactly where in the gut the misfolded proteins come from, or why they form, but some early evidence points to the body’s internal microbial ecosystem. In the latest salvo, scientists from Sweden reported in October that people who had their appendix removed had a lower risk of Parkinson’s years later (SN: 11/24/18, p. 7). The job of the appendix, which is attached to the colon, is a bit of a mystery. But the organ may play an important role in intestinal health.
If the gut connection theory proves true — still a big if — it could open up new avenues to one day treat or at least slow the disease.
“It really changes the concept of what we consider Parkinson’s,” Scheperjans says. Maybe Parkinson’s isn’t a brain disease that affects the gut. Perhaps, for many people, it’s a gut disease that affects the brain.
Gut feeling
London physician James Parkinson wrote “An essay on the shaking palsy” in 1817, describing six patients with unexplained tremors. Some also had digestive problems. (“Action of the bowels had been very much retarded,” he reported of one man.) He treated two people with calomel — a toxic, mercury-based laxative of the time — and noted that their tremors subsided.
But the digestive idiosyncrasies of the disease that later bore Parkinson’s name largely faded into the background for the next two centuries, until neuroanatomists Heiko Braak and Kelly Del Tredici, now at the University of Ulm in Germany, proposed that Parkinson’s disease might arise from the intestine. Writing in Neurobiology of Aging in 2003, they and their colleagues based their theory on autopsies of Parkinson’s patients.
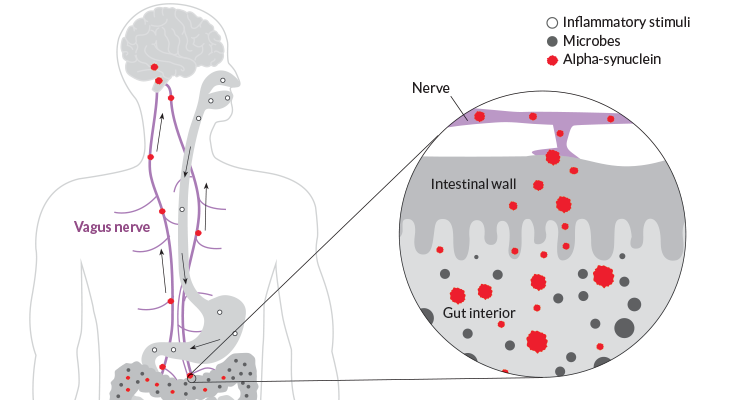
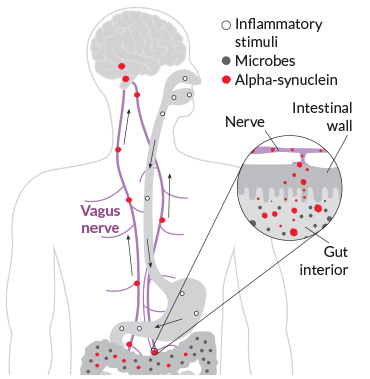
The researchers were looking for Lewy bodies, which contain clumps of a protein called alpha-synuclein. The presence of Lewy bodies in the brain is a hallmark of Parkinson’s, though their exact role in the disease is still under investigation.
Lewy bodies form when alpha-synuclein, which is produced by neurons and other cells, starts curdling into unusual strands. The body encapsulates the abnormal alpha-synuclein and other proteins into the round Lewy body bundles. In the brain, Lewy bodies collect in the cells of the substantia nigra, a structure that helps orchestrate movement. By the time symptoms appear, much of the substantia nigra is already damaged.
Substantia nigra cells produce the chemical dopamine, which is important for movement. Levodopa, the main drug prescribed for Parkinson’s, is a synthetic replacement for dopamine. The drug has been around for a half-century, and while it can alleviate symptoms for a while, it does not slow the destruction of brain cells.
In patient autopsies, Braak and his team tested for the presence of Lewy bodies, as well as abnormal alpha-synuclein that had not yet become bundled together. Based on comparisons with people without Parkinson’s, the researchers found signs that Lewy bodies start to form in the nasal passages and intestine before they show up in the brain. Braak’s group proposed that Parkinson’s disease develops in stages, migrating from the gut and nose into the nerves to reach the brain.
Neural highway
Today, the idea that Parkinson’s might arise from the intestine, not the brain, “is one of the most exciting things in Parkinson’s disease,” says Heinz Reichmann, a neurologist at the University of Dresden in Germany. The Braak theory couldn’t explain how the Lewy bodies reach the brain, but Braak speculated that some sort of pathogen, perhaps a virus, might travel along the body’s nervous system, leaving a trail of Lewy bodies.
There is no shortage of passageways: The intestine contains so many nerves that it’s sometimes called the body’s second brain. And the vagus nerve offers a direct connection between those nerves in the gut and the brain (SN: 11/28/15, p. 18).
In mice, alpha-synuclein can indeed migrate from the intestine to the brain, using the vagus nerve like a kind of intercontinental highway, as Caltech researchers demonstrated in 2016 (SN: 12/10/16, p. 12). And Reichmann’s experiments have shown that mice that eat the pesticide rotenone develop symptoms of Parkinson’s. Other teams have shown similar reactions in mice that inhale the chemical. “What you sniff, you swallow,” he says.
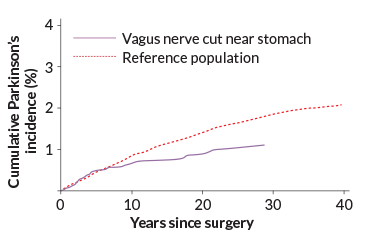
To look at this idea another way, researchers have examined what happens to Parkinson’s risk when people have a weak or missing vagus nerve connection. There was a time when doctors thought that an overly eager vagus nerve had something to do with stomach ulcers. Starting around the 1970s, many patients had the nerve clipped as an experimental means of treatment, a procedure called a vagotomy. In one of the latest studies on vagotomy and Parkinson’s, researchers examined more than 9,000 patients with vagotomies, using data from a nationwide patient registry in Sweden. Among people who had the nerve cut down low, just above the stomach, the risk of Parkinson’s began dropping five years after surgery, eventually reaching a difference of about 50 percent compared with people who hadn’t had a vagotomy, the researchers reported in 2017 in Neurology.
The studies are suggestive, but by no means definitive. And the vagus nerve may not be the only possible link the gut and brain share. The body’s immune system might also connect the two, as one study published in January in Science Translational Medicine found. Study leader Inga Peter, a genetic epidemiologist at the Icahn School of Medicine at Mount Sinai in New York City, was looking for genetic contributors to Crohn’s disease, an inflammatory bowel condition that affects close to 1 million people in the United States.
She and a worldwide team studied about 2,000 people from an Ashkenazi Jewish population, which has an elevated risk of Crohn’s, and compared them with people without the disease. The research led Peter and colleagues to suspect the role of a gene called LRRK2. That gene is involved in the immune system — which mistakenly attacks the intestine in people who have Crohn’s. So it made sense for a variant of that gene to be involved in inflammatory disease. The researchers were thrown, however, when they discovered that versions of the gene also appeared to increase the risk for Parkinson’s disease.
“We refused to believe it,” Peter says. The finding, although just a correlation, suggested that whatever the gene was doing to the intestine might have something to do with Parkinson’s. So the team investigated the link further, reporting results in the August JAMA Neurology.
In their analysis of a large database of health insurance claims and prescriptions, the scientists found more evidence of inflammation’s role. People with inflammatory bowel disease were about 30 percent more likely to develop Parkinson’s than people without it. But among those who had filled prescriptions for an anti-inflammatory medication called antitumor necrosis factor, which the researchers used as a marker for reduced inflammation, Parkinson’s risk was 78 percent lower than in people who had not filled prescriptions for the drug.
Belly bacteria
Like Inga Peter, microbiologist Sarkis Mazmanian of Caltech came upon Parkinson’s disease almost by accident. He had long studied how the body’s internal bacteria interact with the immune system. At lunch one day with a colleague who was studying autism using a mouse version of the disease, Mazmanian asked if he could take a look at the animals’ intestines. Because of the high density of nerves in the intestine, he wanted to see if the brain and gut were connected in autism.
Neurons in the gut “are literally one cell layer away from the microbes,” he says. “That made me feel that at least the physical path or conduit was there.” He began to study autism, but wanted to switch to a brain disease with more obvious physical symptoms. When he learned that people with Parkinson’s disease often have a long history of digestive problems, he had his subject.
Mazmanian’s group examined mice that were genetically engineered to overproduce alpha-synuclein. He wanted to know whether the presence or absence of gut bacteria influenced symptoms that developed in the mice.
The results, reported in Cell in 2016, showed that when the mice were raised germ free — meaning their insides had no microorganisms — they showed no signs of Parkinson’s. The animals had no telltale gait or balance problems and no constipation, even though their bodies made alpha-synuclein (SN: 12/24/16 & 1/7/17, p. 10). “All the features of Parkinson’s in the animals were gone when the animals had no microbiome,” he says.
However, when gut microbes from people diagnosed with Parkinson’s were transplanted into the germ-free mice, the mice developed symptoms of the disease — symptoms that were much more severe than those in mice transplanted with microbes from healthy people.
Mazmanian suspects that something in the microbiome triggers the misfolding of alpha-synuclein. But this has not been tested in humans, and he is quick to say that this is just one possible explanation for the disease. “There’s likely no one smoking gun,” he says.
Microbial forces
If the microbiome is involved, what exactly is it doing to promote Parkinson’s? Microbiologist Matthew Chapman of the University of Michigan in Ann Arbor thinks it may have something to do with chemical signals that bacteria send to the body. Chapman studies biofilms, which occur when bacteria form resilient colonies. (Think of the slime on the inside a drain pipe.)
Part of what makes biofilms so hard to break apart is that fibers called amyloids run through them. Amyloids are tight stacks of proteins, like columns of Legos. Scientists have long suspected that amyloids are involved in degenerative diseases of the brain, including Alzheimer’s. In Parkinson’s, amyloid forms of alpha-synuclein are found in Lewy bodies.
Despite amyloids’ bad reputation, the fibers themselves aren’t always undesirable, Chapman says. Sometimes they may provide a good way of storing proteins for future use, to be snapped off brick by brick as needed. Perhaps it’s only when amyloids form in the wrong place, like the brain, that they contribute to disease. Chapman’s lab group has found that E. coli bacteria, part of the body’s normal microbial population, produce amyloid forms of some proteins when they are under stress.
When gut bacteria produce amyloids, the body’s own cells could also be affected, wrote Chapman in 2017 in PLOS Pathogens with an unlikely partner: neurologist Robert Friedland of the University of Louisville School of Medicine in Kentucky. “This is a difficult field to study because it’s on the border of several fields,” Friedland says. “I’m a neurologist who has little experience in gastroenterology. When I talked about this to my colleagues who are gastroenterologists, they’ve never heard that bacteria make amyloid.”
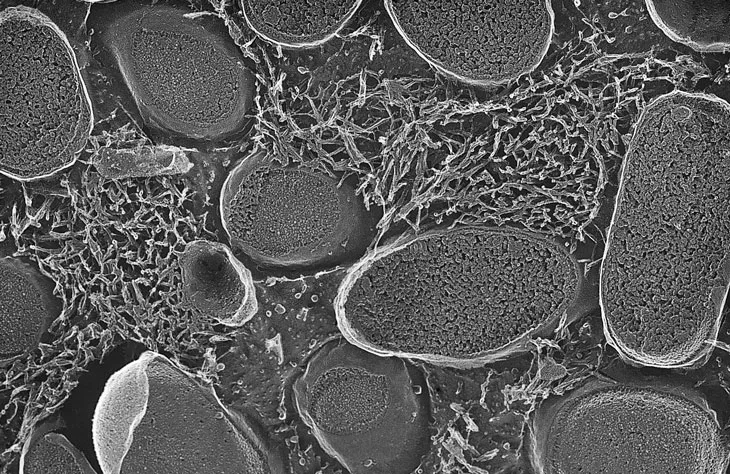
Friedland and collaborators reported in 2016 in Scientific Reports that when E. coli in the intestines of rats started to produce amyloid, alpha-synuclein in the rats’ brains also congealed into the amyloid form. In their 2017 paper, Chapman and Friedland suggested that the immune system’s reaction to the amyloid in the gut might have something to do with triggering amyloid formation in the brain.
In other words, when gut bacteria get stressed and start to produce their own amyloids, those microbes may be sending cues to nearby neurons in the intestine to follow suit. “The question is, and it’s still an outstanding question, what is it that these bacteria are producing that is, at least in animals, causing alpha-synuclein to form amyloids?” Chapman says.
Head for a cure
There is, in fact, a long list of questions about the microbiome, says Scheperjans, the neurologist whose paper Martha Carlin first spotted. So far, studies of the microbiomes of human patients are largely limited to simple observations like his, and the potential for a microbiome connection has yet to reach deeply into the neurology community. But in October, for the second year in a row, Scheperjans says, the International Congress of Parkinson’s Disease and Movement Disorders held a panel discussing connections to the microbiome.
“I got interested in the gastrointestinal aspects because the patients complained so much about it,” he says. While his study found definite differences in the bacteria of people with Parkinson’s, it’s still too early to know how that might matter. But Scheperjans hopes that one day doctors may be able to test for microbiome changes that put people at higher risk for Parkinson’s, and restore a healthy microbe population through diet or some other means to delay or prevent the disease.

One way to slow the disease might be shutting down the mobility of misfolded alpha-synuclein before it has even reached the brain. In Science in 2016, neuroscientist Valina Dawson and colleagues at Johns Hopkins University School of Medicine and elsewhere described using an antibody to halt the spread of bad alpha-synuclein from cell to cell. The researchers are working now to develop a drug that could do the same thing.
The goal is to one day test for the early development of Parkinson’s and then be able to tell a patient, “Take this drug and we’re going to try to slow and prevent progression of disease,” she says.
For her part, Carlin is doing what she can to speed research into connections between the microbiome and Parkinson’s. She quit her job, sold her house and drained her retirement account to pour money into the cause. She donated to the University of Chicago to study her husband’s microbiome. And she founded a company called the BioCollective to aid in microbiome research, providing free collection kits to people with Parkinson’s. The 15,000 microbiome samples she has collected so far are available to researchers.
Carlin admits that the possibility of a gut connection to Parkinson’s can be a hard sell. “It’s a difficult concept for people to wrap their head around when you are taking a broad view,” she says. As she searches for answers, her husband, John, keeps going. “He drives, he runs biking programs in Denver for people with Parkinson’s,” she says. Anything to keep the wheels turning toward the future.