A monkey survived two years with a miniature pig’s kidney
Researchers genetically modified pigs to make their organs more usable for human transplantation one day
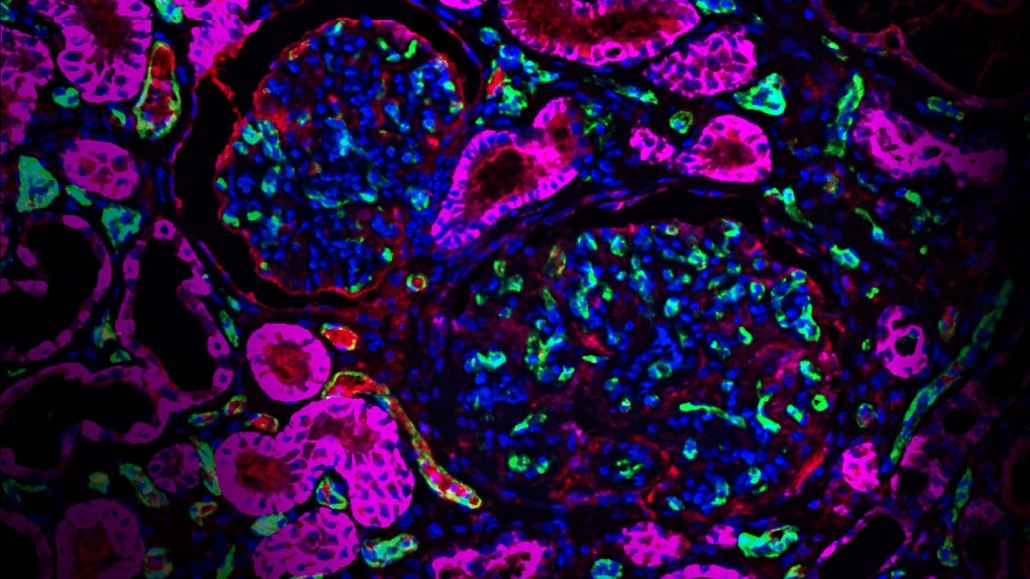
Scientists genetically engineered pigs with kidneys that produced human proteins, shown in fuchsia. Such modifications may one day help people’s bodies accept an organ transplant from a different species.
Violette Paragas, eGenesis
Little pigs with big genome edits could be the future of organ donation.
A monkey that received a kidney from a genetically engineered miniature pig lived for more than two years after the transplant, scientists report October 11 in Nature. The team took a molecular red pen to donor pigs’ genomes, editing the animals’ organs to be more of a match for humans. Such an editing strategy could one day make it more likely for people’s bodies to accept organs from different species.
The work, funded by biotechnology company eGenesis, is the latest in a string of efforts seeking to use other species to address global organ shortages. The new study is “promising work and a step in the right direction,” says Parsia Vagefi, a transplant surgeon at UT Southwestern Medical Center in Dallas, who was not involved with the research.
In the United States, the demand for new organs to replace damaged or diseased ones far outpaces supply. As of October 11, nearly 104,000 Americans sit on the national transplant waiting list. Some 89,000 of these people are seeking kidneys. “There just simply aren’t enough kidneys to go around,” said eGenesis president and CEO Mike Curtis in a news conference on October 10. Most people waiting will never get the offer, he says. “They will die on dialysis.”
That’s where cross-species transplantation — giving people organs from other animals — comes in, Curtis said. It’s “the only near-term viable solution to solving this huge shortfall in organ availability.”
But the path forward is studded with scientific stumbling blocks. Pig organs aren’t a molecular match for human bodies, for one. Porcine cells display suspicious-looking sugars that spell “stranger” to our immune systems. This can prompt rejection of a transplanted organ.
If the body sees those sugars, “It’s like, ‘whoa, you’re not supposed to be here,’ and the immune system attacks,” says Jayme Locke, a transplant surgeon at the University of Alabama at Birmingham. Scientists tweak donor animals’ genomes to “make the pig kidney as human as possible so that our body recognizes it and doesn’t immediately attack it,” she says.
Locke and others have already made progress in this field. In 2021, a team at NYU Langone Health tested how well a kidney from a genetically modified pig functioned by attaching it to a brain-dead woman (SN: 10/22/21). In 2022, Locke’s group upped the ante, publishing the first peer-reviewed study on a gene-edited pig kidney transplanted into a brain-dead person.
That year, doctors also transplanted modified pig hearts into two brain-dead people — and even a living man (SN: 7/12/22). The man, 57-year-old David Bennett from Maryland, survived for two months with the transplanted organ (SN: 1/31/22). This September, another patient, Lawrence Faucette, became the second living person to receive a genetically modified pig heart.
In August, Locke’s team reported that pig kidneys transplanted into a brain-dead man can function normally, producing urine over the course of a seven-day study. Her team worked with a different kind of genetically modified pig than the ones Curtis and his colleagues created — one with 10 genomic edits rather than up to 69.
The edits, which strip away some pig aspects and add in human ones, fall into three main categories designed to make the animals’ organs safer for human use. They lop off stranger-danger sugars from pigs’ cells, insert human genes to help prevent organ rejection and knock out potentially harmful retroviruses embedded in the pigs’ genomes. “This is probably the most genetically modified pig that I’ve seen described in the literature,” Locke says.
Curtis’ team mixed and matched edits in different pigs and transplanted their kidneys into monkeys. Because these are pig kidneys genetically tailored for humans, scientists have to dose the monkeys with powerful immunosuppressive drugs, so the animals don’t reject the new organs. But these types of animal studies are important, Locke says, because they can demonstrate long-term organ function, which is difficult to show in people with brain death.
Eight out of 15 monkeys that received pig kidneys lacking the sugars and carrying the human genes survived 176 days or longer, Curtis’ team showed. One monkey lasted much longer, living 758 days after the kidney transplant. Monkeys with pig kidneys missing the human genes didn’t fare nearly as well, surviving a median of 24 days.
“It’s encouraging when you see a pig kidney being able to function for two years,” Vagefi says. “But I think it’s even more encouraging when you see it done consistently.” Like any good study, he says, the work opens a chasm of questions. He wonders, for example, how many more genetic edits pig organs may need to succeed long-term in monkeys regularly.
Even then, there might not be a single kind of pig donor that’s right for every person, Locke points out. She thinks it’s important to have options. Doctors may “need more than one type of genetically edited pig in order to serve all the people in need.”