A rare mutation helped one man stave off Alzheimer’s for decades
It’s the second such case ever to be reported
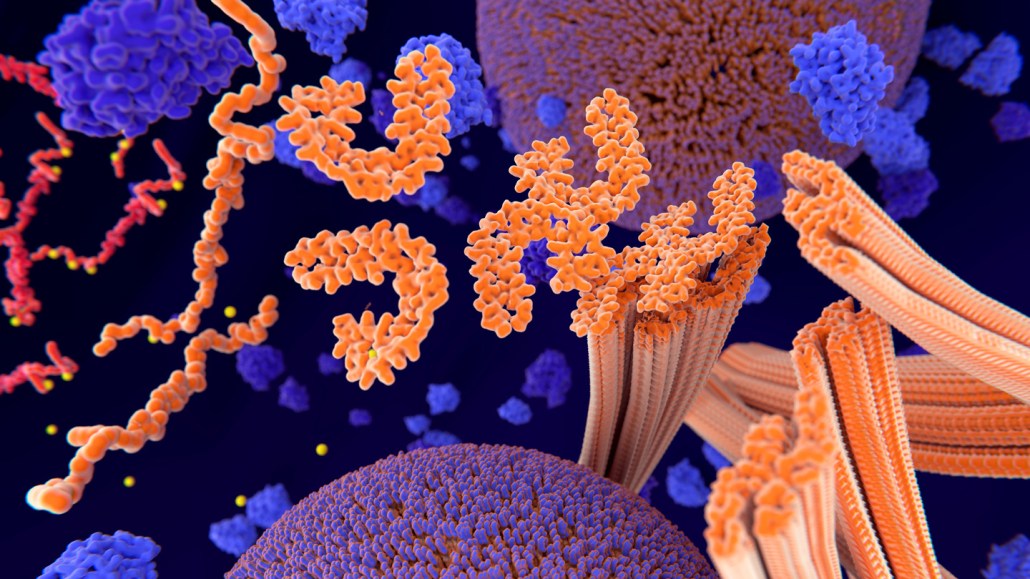
Aggregations of proteins called tau tangles (orange clumps in this illustration of a brain cell) are one of the major signs of Alzheimer's disease.
JUAN GAERTNER/SCIENCE PHOTO LIBRARY/Getty Images Plus
A rare genetic mutation never seen before protected a man with an inherited form of Alzheimer’s from developing the disease for decades.
He is the second person found to have such protection, following a report in 2019 of a woman with a different mutation (SN: 1/26/20). Both mutations may have staved off the disease for years by acting in similar ways in the brain, an insight that could lead to new treatments for all forms of Alzheimer’s, scientists report May 15 in Nature Medicine.
But some researchers are cautious about concluding too much from just two cases. “The results look very promising, but it would be useful to see replication in more samples,” says neurologist Rudolph Tanzi of Harvard Medical School who was not involved in the new study. Still, the work is important as “it can serve as a useful guide for drug discovery,” he says.
Both the man and woman were members of a Colombian family who have a mutation in the PSEN1 gene that causes the rare inherited variety of Alzheimer’s. People with “familial” Alzheimer’s usually start showing signs in their 40s. The more common “sporadic” form does not cause symptoms until people are in their 70s or 80s.
The woman stayed sharp into her 70s, while the man described in the new study was still mentally healthy at 67. “That means they were protected, because they should have gotten the disease 30 years earlier, and they didn’t,” says Diego Sepulveda-Falla, a neurologist at the University Medical Center Hamburg-Eppendorf in Germany.
The woman had a protective mutation in a gene closely linked to Alzheimer’s, APOE. This mutation is known as the Christchurch variant, after the city in New Zealand where it was first found. The mutation identified in the new study was in a gene called RELN. Sepulveda-Falla and colleagues named this new mutation RELN-COLBOS, after a joint Colombia-Boston study that the man participated in. He died three years ago, at age 74, from other causes, and his family donated his brain for study.
The researchers compared the two cases, finding striking similarities and differences. Amyloid plaques, thought by many researchers to be deeply involved in Alzheimer’s, were abundant in both patients’ brains. But the woman had low levels of another possible Alzheimer’s culprit, clusters of proteins called tau tangles. The researchers think this is what spared her from dementia for decades, as tau is more tightly linked to symptoms than amyloid, researchers suspect.
In the Colombian man’s brain, the researchers found a different picture for tau. “Unlike the Christchurch case, this case was severely affected by tau,” Sepulveda-Falla says. “This shocked us initially, then we figured out we needed to roll our sleeves up and dig deeper.”
Some brain regions, notably the entorhinal cortex, which is important for memory and one of the earliest areas affected in Alzheimer’s, had been spared from the tau buildup, the team found.
The difference in tau between the two cases is due to where the two protective genes are active in the brain. In adults, RELN is active in only a few places, including the entorhinal cortex. APOE is active everywhere. “Since APOE is ubiquitous, in one patient you get protection all around,” Sepulveda-Falla says. “In this other, the protection is localized to [certain] neurons, and by chance they happen to be the neurons that are key for preserving cognition.”
Despite affecting different genes, both mutations produce proteins that attach to the same molecules on cells, and, ultimately, appear to reduce the formation of tau tangles. Sepulveda-Falla and colleagues confirmed this for the new mutation using mice genetically engineered to produce tau. Introducing the RELN-COLBOS mutation into these mice prevented tau buildup. This mechanism, common to both mutations, could be targeted by new treatments aiming to stave off all types of Alzheimer’s, the researchers say.