Residents of the brain
Scientists turn up startling diversity among nerve cells
Peer out the window of a plane landing at LaGuardia Airport, and the tiny people scurrying around the streets of New York City all look the same. But take a stroll down Fifth Avenue and a new view emerges: Up close, New Yorkers are very different.
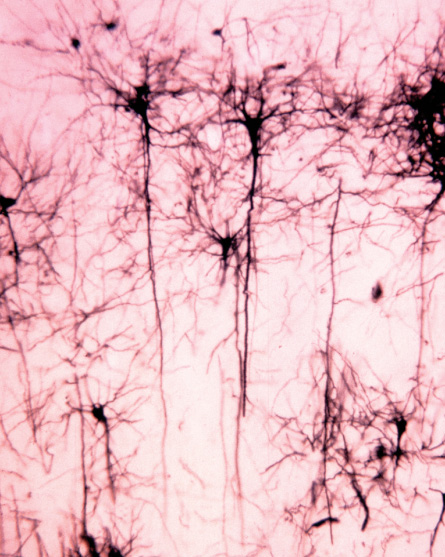
A street view of the brain also reveals a new perspective: No two cells are the same. Zoom in, and the brain’s wrinkly, pinkish-gray exterior becomes a motley collection of billions of cells, each with personalized quirks and idiosyncrasies.
Powerful new techniques are giving researchers a glimpse of this staggering diversity — especially among nerve cells, the brain’s information brokers. Even nerve cells presumed to do the same job come in a range of shapes and sizes and display a host of behaviors, sending their electrical messages in unpredictable ways, new studies reveal. The closer scientists scrutinize nerve cells, called neurons, the more differences turn up.
This cellular menagerie has left researchers puzzling over how best to categorize what neuroscientist Rafael Yuste of Columbia University calls these “living creatures.” So far, systematic methods are lacking. “Even after 100 years of research, we have no clue how many classes of neurons there are,” says Yuste, a Howard Hughes Medical Institute researcher. He and other scientists are developing new algorithms to automate neuron classification, in the hope of someday compiling a standard “parts list” of the brain.
While some scientists are hard at work categorizing all these different cells, others are thinking about what such diversity means for living, breathing animals. New results suggest, for instance, that a population of nerve cells in which individual responses to an electrical poke differ can process more information than a group in which responses are the same. Others think that variety might help the brain cope with a changing environment.
Accounting for all of the individual brain components — a task as daunting as finding out every New Yorker’s favorite color, credit score and whether they cry at sad movies — isn’t just a tedious sorting job. A deeper knowledge of the brain’s inhabitants might lead to new treatments for brain-related disorders. If particular cells are more vulnerable to diseases such as dementia, schizophrenia and autism, therapies that protect or target these cell populations may be effective. More broadly, knowing who is doing what in the brain will help scientists understand the inner workings of the impossibly complex three-pound hunk of flesh that sits in the skull.
Brain census
Yuste is a collector. Instead of Broadway ticket stubs or rare butterflies, he and his colleagues sift through nerve cells in the brain, meticulously cataloging each cell’s appearance, behavior and habitat. Yuste believes that before scientists can understand the boroughs of the brain, they must know who lives there. “People like us are interested in studying how the brain works by taking it apart,” he says.
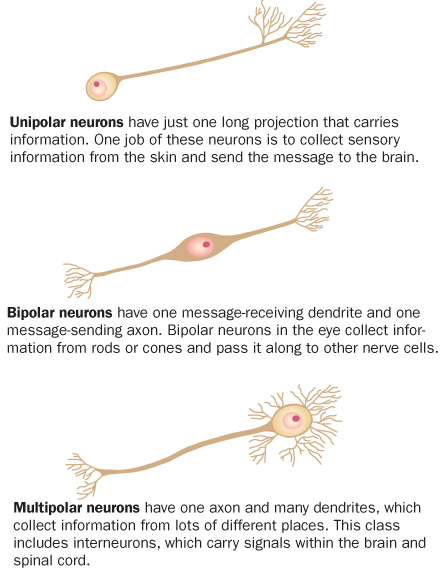
Such efforts have described what appears to be a brain teeming with different groups of neurons. By virtue of their job description, these chief information officers have a message-sending axon and message-receiving dendrites, but that’s about where the similarities end.
Consider the pine needle–like extensions of granule neurons, which help memories form deep in the brain; the behemoth, feathery Purkinje neurons that coordinate body movements; and the twinkly stellate interneurons that help transmit visual signals.
In the past, such cell groups were uncovered by careful observation followed by whimsical naming. (How else to explain the “chandelier neuron”?) Yet this hodgepodge approach can go only so far. “What people have done traditionally is make up classifications,” Yuste says. “They look at neurons and say, ‘Oh, this neuron looks different from this other neuron.’ So they call this neuron class A and that neuron class B. This has led nowhere.”
He and his colleagues advocate a more systematic way. So far, the team has compiled descriptions of the traits of about 1,000 individual neurons. With informative descriptions available, computer algorithms and statistical tests put these cells into more orderly — and meaningful — classes. Like sophisticated coin sorters, these algorithms can consider loads of different neuron measurements, everything from body size and branch pattern to how fast electrical messages are sent. The team is currently testing computer-based sorting programs in the hopes that they will be less capricious than a single person’s eyeballs.
Recently, Yuste and colleagues used such methods to identify two new “subtypes” of cells called interneurons in the mouse neocortex, the outer layer of the brain that controls, among other things, sensory perception. In the study, researchers led by Laura McGarry of Columbia reconstructed the sizes and shapes of 59 individual neurons, and also characterized the electrical pulses that the neurons use to communicate among themselves. This careful scrutiny resulted in a long list of properties, which was then fed into a computer. An algorithm divvied up the 59 neurons into three groups, two of which had never before been described, the team reported last year in Frontiers in Neural Circuits.
Cells belonging to the third subgroup, called Martinotti cells, had far-reaching and elaborate branching patterns, while cells in the two new subgroups seemed to be less spread out, McGarry says. The new groups showed subtle differences in the way they pass information. More experiments are needed to see whether this signal-sending variation is important.
Neuroscientist Kristin Baldwin is looking not at signals themselves, but at the precise shape of the long, spidery axons that send them. Baldwin, of the Scripps Research Institute in La Jolla, Calif., and her colleagues figured out a way to label individual mouse neurons with molecules that serve as beacons, glowing brightly when hit with particular wavelengths of light. Not that it was easy.Following the projections of a single nerve cell initially took about six weeks.
The particular neurons Baldwin studies, called mitral cells, are smell go-betweens, carrying odor information from scent-detecting cells in the nose to higher brain regions. Because mitral cells are situated close to each other and have the same job description, Baldwin expected their info-sending axons to look similar. Instead, the location, shape and numbers of axon branches appear very different from neuron to neuron, the team reported in the April 14 Nature. Preliminary studies suggest that these cells are in fact transmitting information to very different targets in the brain.
Baldwin and her colleagues would next like to trace mitral cells into the amygdala, a brain region involved in emotion, to see what the axons look like there.
Staring at the shapes of single neurons has convinced Baldwin that “the harder you try to find two neurons that are the same, the more difficult it becomes.” The true diversity of the brain may be greater than anyone anticipated, she says.
Information overload
It was the bizarrely unpredictable and diverse behavior of mitral cells that alerted Nathan Urban to the possibility that variety might help the brain do its job. Urban’s tip-off came from working with postdoc Krishnan Padmanabhan, who was learning how to record the electrical activity of these mouse neurons.
Urban remembers Padmanabhan coming into his office at Carnegie Mellon University in Pittsburgh saying, “I think I did something wrong. The cells’ responses look different today.” As someone who had studied those cells for years, Urban could reassure his colleague, saying, “No, it’s OK. Sometimes they look like that.” When Padmanabhan came back the next day with totally different data, Urban reassured him again: “Yeah, sometimes they look like that, too.”
This constant barrage of variation got Urban wondering whether there was an important brain principle at work. Maybe this variety results because the brain isn’t able to make identical neurons, Urban remembers thinking. But then again, maybe there’s something useful about having a wide repertoire of behavior in this neuron class.
“When we started thinking about if this was a feature rather than a bug, that’s when we started to get really excited about it,” Urban says.
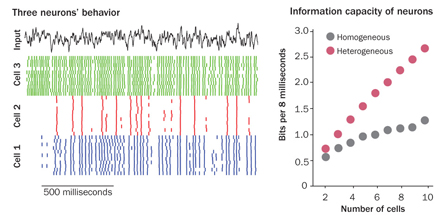
A group of neurons that respond differently to the exact same input — in this case, a slight electrical jolt calibrated to be exactly the same for each cell — can actually transmit more information than cells that all respond exactly the same way. In their study, Padmanabhan and Urban converted cells’ electrical responses into binary information, a series of 0s and 1s. Cell populations with diverse responses could carry twice as much information as cells that all behaved the same, the team reported last October in Nature Neuroscience. The results suggest that a varied group of eight of these smell-related cells could carry about 24.5 bits of information per sniff, while a group of eight cells that all responded in concert could carry only about 12.6 bits.
“This is a new idea, really,” Urban says, “that within what would normally be considered to be one class of cells, there might be variation that is useful to the system, to the organism.”
Instead of just speculating about the function of diversity, Urban and his colleagues are testing it by simulating virtual cohorts of neurons. Tweaking the degrees of variation has serious consequences for the group’s behavior, Urban and his team reported February 25 at the Computational and Systems Neuroscience Meeting in Salt Lake City.
“When the neurons are very similar to each other, you enter states where all the neurons are doing the same thing, and that’s useless,” Urban says. “If you have all the neurons in your brain doing the same thing, you’re unconscious or you’re dead. That’s not a desired state.”
By playing with these models, Urban and his colleagues have come to view diversity as a commodity, something that’s probably precisely tuned to lend a hand to whatever task needs doing. They are looking to other brain regions, such as the memory-encoding hippocampus, for signs that diversity is a universal perk.
In the animal
Tallying up the various classes, types and groups of neurons in the brain, and understanding how those neurons help encode extra information, is incredibly hard, says neuroscientist Chris Moore of Brown University in Providence, R.I. But the ultimate goal is even more difficult — figuring out how the diversity contributes to the behavior of a mouse sniffing around for food while avoiding a cat. “That’s a tall order,” Moore says.
Last year, Moore and his colleagues wrote an essay in Cell (subtitle: “From diversity, strength”) exploring the various ways that diversity among brain cells might contribute to perception. The idea is that a diverse group of neurons could help the brain reliably recognize the same thing under a range of circumstances. “Whether it’s a bright sunny day, or a dark, cloudy day, you can still recognize your grandmother,” Moore says.
This ability to consistently recognize grandma in all sorts of lighting scenarios, or even in a grainy old photograph, might be a result of differences among neurons. Moore calls this “diversity for consistency’s sake.” Alternatively, neuron diversity might be behind the brain’s ability to flexibly assess different, unexpected things (or, as Moore puts it, “diversity for diversity’s sake”). Experiments are needed to sort out whether diversity helps in either — or neither, or both — of these scenarios.
Moore and his team are undertaking such experiments and turning up hints of potentially meaningful differences among the same interneurons that Yuste and McGarry study. A steady stream of information coming into the senses seems to spur some interneurons into action, while others couldn’t care less, preliminary studies from Moore’s lab show. Eventually, these disparate patterns of activity might help scientists decode perception, and more generally, mouse — or even human — behavior.
Understanding this variation might also have implications for neurological diseases. Diseases that cause neuron death, for instance, might selectively attack certain kinds of cells first. “What if some neurons are more sensitive to certain kinds of damage?” Urban says. “Is it a random subset, or is it something that’s a more insidious kind of damage?” Perhaps certain cells are extra-vulnerable to a stroke, or disease such as Alzheimer’s, or even normal aging.
If that’s true, then in addition to losing neurons, the brain would lose diversity, a deficit that could usher in even more damage. So far, this idea has some hints of support: In Urban’s simulations, a loss of diversity leads to “bizarre, pathological kinds of behavior” in networks of cells. And the loss of particular kinds of neurons has been tied to schizophrenia and Parkinson’s. A study published online June 8 in Cerebral Cortex shows that two specific kinds of neurons, fork cells and von Economo neurons, die early in people with a particular type of dementia. Researchers might be able to develop ways to protect the most vulnerable cells if they can figure out why some neurons succumb to certain diseases.
Diverse models of diversity
To uncover the hidden variety among such neurons, scientists would do well to take a suggestion from the neurons themselves, and diversify. The traditional way to capture neuron activity involves a single, finely tuned set of mathematical equations. These equations try to explain a cell’s behavior — the speed and strength of electrical signals, for instance — by averaging info from a group of cells. But a collection of models, each with its own strengths and weaknesses, may offer better results, says neuroscientist Eve Marder of Brandeis University in Waltham, Mass.
Single mathematical models are often geared toward catching the “Platonic ideal” of an individual neuron’s behavior, yet any biologist conducting experiments knows that no such thing exists, Marder writes online March 7 in the Proceedings of the National Academy of Sciences. Seeing fluctuations among neuron behavior in the lab, Marder says, “eventually led to a whole different way of thinking about how to build models and how variable the underlying biology would have to be, or could be, or should be.”
Messy biological data call for diverse models. “If you assume that there’s a single output and a single solution, you may be missing really, really deep biological mechanisms,” she says.
These deep biological mechanisms, perhaps including diversity, might explain not only how the brain can operate so smoothly, but also how the brain came to be, too. Neurons with different sets of capabilities, looks and behavior invite natural selection in to sculpt the brain, nurturing cells that are helpful and pruning the ones that aren’t. Diversity may be the reason humans ended up with big brains in the first place. After all, as Marder points out, “Variability is a really great substrate for evolution to work on.”
Of course, it’s not like the brain had a choice, Marder says. The brain can’t make perfect neurons that roll off the assembly line with exact specifications. So for now, whether diversity among neurons is an inescapable bug that results from messy biology or a feature of a powerful, flexible brain may simply be irrelevant. The important thing is that this diversity exists, and appreciating it fully may bring scientists closer to understanding the mysterious brain in all of its wild glory.







