
Stentor roeseli, a single-celled organism known as a protist, uses its hairlike cilia to feed while attached to floating algae. New research suggests that it's capable of complex evasive behaviors when irritated.
Proyecto Agua/Flickr (CC BY-NC-SA 2.0)
Being single-celled doesn’t necessarily doom a creature to a simple life. A fresh look at a long-dismissed, century-old experiment suggests that so-called primitive organisms can behave in surprisingly complex ways.
Stentor roeseli, a tiny trumpet-shaped protist, can dodge, duck or flee in response to an irritating stimulus, changing its behavior when one strategy fails, researchers report online December 5 in Current Biology. The study suggests that single cells, rather than being preprogrammed to react in a certain way, are capable of “changing their minds” based on experience.
“This fascinating experiment reminds us that primitive organisms can do complicated things,” says Sindy Tang, a cellular engineer at Stanford University who wasn’t involved in the study.
S. roeseli rose to prominence in 1906, when the American zoologist Herbert Spencer Jennings described some of the most complex behaviors ever reported for a single-celled organism. The millimeter-long freshwater protist spends much of its life fastened to drifting algae, using hairlike cilia on its body to sweep food into its mouth.
Jennings messed with S. roeseli, disturbing them with a pipette-delivered stream of a chemical irritant. Instead of simple reflexive behaviors, he documented a complex hierarchy of avoidance tactics. First, the protist would bend to dodge the onslaught. If that failed, it would repel the irritant by using its cilia to “spit” water out of its mouth. When Jennings persisted, it would contract its whole body to shrink away. Its final act was to escape by detaching from its substrate and floating away.
At the time, biologists considered single cells to be capable only of rudimentary behaviors, such as moving toward or away from some stimulus. Consequently, Jennings’ work garnered much attention. But attempts to replicate it failed, and eventually his observations were dismissed.
But when Jeremy Gunawardena, a systems biologist at Harvard Medical School in Boston, learned of Jennings’ work from a colleague’s lecture, “I was surprised and immediately fascinated,” he says. “It suggested that single cells can have a sort of autonomy that we don’t consider nowadays.” Gunawardena tracked down some of the major replication studies and noticed a major flaw: They all used a different species of Stentor with a more mobile lifestyle than S. roeseli.
With the right species in hand, Gunawardena and his colleagues set out to replicate the century-old experiment. Instead of chemicals, they shot pulses of tiny plastic beads at S. roeseli each time the cells appeared to be in a resting state and recorded their behaviors.
Over 57 experiments, the researchers observed each of the behaviors first described by Jennings, but noted substantially more variability than in the original experiment. Some of the cells repeated the same steps or skipped some altogether.
Evasive maneuvers
Stentor roeseli exhibits a range of evasive behaviors when irritated, as seen in this drawing based on the observations reported in 1906 by American zoologist Herbert Spencer Jennings. From a resting position, during which its cilia are sweeping water and food toward its mouth, the single-celled organism can bend away from an irritant, reverse the direction of its cilia to “spit” the irritant out, contract, or detach and float away.
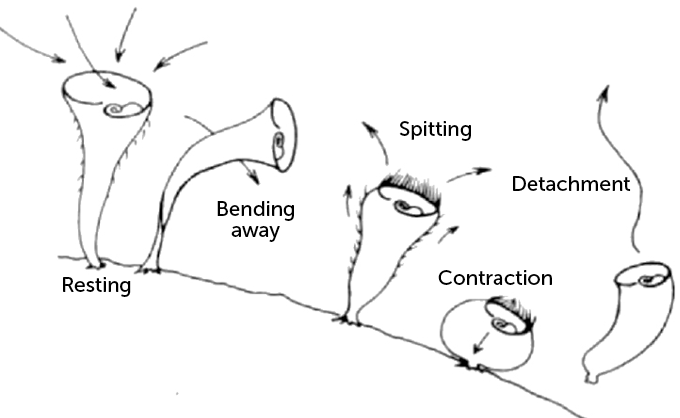
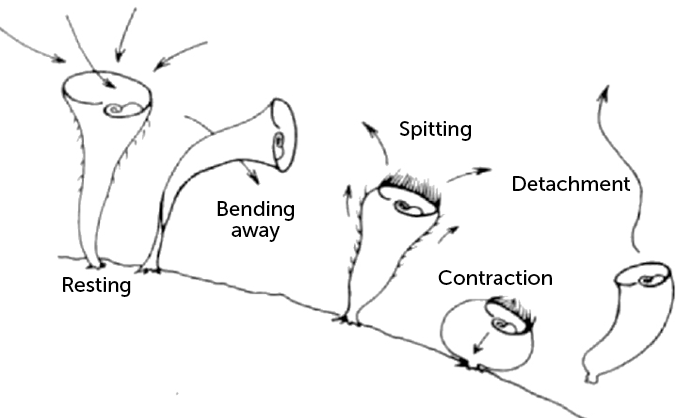
Initially the researchers were puzzled. But when they analyzed the behaviors of all S. roeseli used in the experiments, a hierarchy emerged. More often than not, an irritated S. roeseli cell will first bend away or try to spit out the beads. Then, it will either contract or detach, but the researchers never saw a cell detach without contracting first.
These results suggest that S. roeseli can, in a sense, change its mind about how to respond to an irritant, the researchers say. “We showed that a single cell is capable of fairly sophisticated decision-making,” Gunawardena says.
The team was especially surprised to find that, after contracting once, there’s a 50-50 chance that S. roeseli will either contract again or detach. That’s a decision-making process that seems akin to flipping a coin. Such unpredictability could give S. roeseli an advantage in keeping predators on their toes, Gunawardena says.
Kirsty Wan, a biophysicist at the University of Exeter in England, welcomes revisiting Jennings’ work. It’s “a great start toward understanding how these particular cells make decisions,” she says. Using software to denote certain behavioral states, instead of relying on a researcher’s subjective assessment, could strengthen future studies, she says.
Gunawardena hopes this study will push biologists to think about cells differently. Rather than being genetically programmed to respond uniformly to some stimulus, he says individual cells might instead be programmed with “machinery that allows the cell to have some autonomy about what it does depending on the context.”







