A Soft Touch: Imaging technique reveals hidden atoms
One of today’s celebrity scientific instruments, the atomic force microscope (AFM), is valued despite some quirks. Famous for rendering atoms visible, it can also be blind.
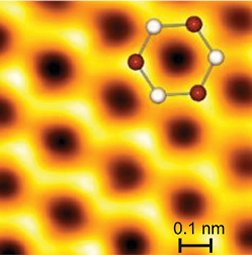
That shortfall has been particularly glaring when it comes to graphite. AFM images reveal only three of the six carbon atoms in each of the material’s basic hexagonal units. In an upcoming Proceedings of the National Academy of Sciences, a team of German physicists describes how it solved that problem. The advance may lead to techniques to image biological materials, the physicists say.
In graphite, the hexagonal units fuse into sheets resembling miniaturized chicken wire. Loose connections between these sheets make graphite soft; it’s these sheets that a pencil leaves behind on paper. When intact, the sheets stack such that every other carbon in each ring rests directly above a carbon in the sheet below. These are known as alpha atoms. The other carbons, called beta atoms, have nothing directly underneath.
When the AFM’s cantilever tip passes over the graphite, it gently tugs on each carbon atom but can detect the attractive forces only between the tip and the beta atoms. That’s because electrons in the alpha atoms overlap with those of the atoms below, restricting interactions between the electrons and the AFM tip. In contrast, the less-fettered electrons of the beta atoms show up in AFM images.
Jochen Mannhart and his colleagues at the University of Augsburg in Germany modified their AFM to measure repulsive forces instead of attractive ones. The tip pushes down on each atom “like an atomic braille system,” explains Yip-Wah Chung at Northwestern University in Evanston, Ill.
The researchers needed to make sure the AFM tip wasn’t pushing down on the graphite surface too forcefully. “Otherwise, the carbon [atom] will disappear inside the material,” says Mannhart.
As the tip approaches a carbon atom, the electron clouds and the tip repel each other, changing the cantilever’s vibration frequency. In this mode, both alpha and beta atoms become visible.
The procedure is slow. To prevent subtle motions in the sample and instrument that would blur the images, the measurements must be carried out at just a few degrees above absolute zero.
Other types of microscopes can image hard, electrically conducting materials with atomic resolution, but soft, nonconducting materials such as graphite and biological molecules have been difficult to image.
To probe DNA and proteins, says Northwestern’s Mark Hersam, the German technique needs to be modified so that it works at much warmer temperatures that preserve the samples’ biologically relevant structures.
****************
If you have a comment on this article that you would like considered for publication in Science News, send it to editors@sciencenews.org. Please include your name and location.







