Solar panels to dye for
Scientists show that cheap chemical dyes may lead to efficient conversion of the sun's energy
If you think solar is still too expensive, here’s how to get more bang for your solar-cell buck.
Take a small solar cell, and slice it into thin slivers. Wrap the slivers around the edges of a slab of glass. Paint the glass with a high-tech, but cheap, dye — which you bought online — and voilà! You have a new solar panel. It can collect much more energy than the pricey cell you started with, and it costs only a little more.
If done right, making solar panels with a new generation of dyes complementing conventional photovoltaic cells would be cheaper and thus more competitive with other energy sources, Marc Baldo of the Massachusetts Institute of Technology and his colleagues report in the July 11 Science. Already in the team’s lab demonstration, “it gives a tenfold increase in total power per unit area of conventional photovoltaic,” Baldo says.
The team’s results show that the technology can potentially reduce the cost of solar panels, comments Sean Shaheen of the University of Denver. “Whether or not a tenfold cost reduction would be possible in a large-scale manufacturing effort is an open question.”
The most efficient solar cells are made from crystals of a semiconductor such as silicon. Once installed, solar cells give out energy for free. But silicon crystals are expensive to produce, so solar cells can take decades to pay for themselves.
One strategy to deal with this issue follows from the fact that solar cells become more efficient when concentrated light shines on them. Engineers are experimenting with parabolic mirrors to reflect light onto small, high-power cells. But to work well, such mirrors must point at the sun all the time, so they need sophisticated tracking mechanisms that keep turning them throughout the day.
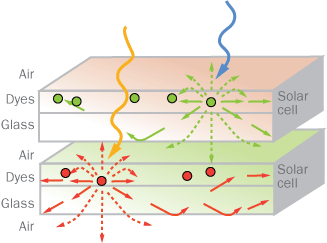
Baldo and his colleagues looked to dyes for a simpler approach: They gather light with dye on a glass surface and then concentrate it on the much smaller surface area of the glass’s edges. The dye, painted on the glass, absorbs sunlight and then gives it back via fluorescence. Photons come out of dye molecules in random directions, with some 80 percent shooting out horizontally or bouncing off the inner surface of the glass at an angle. That way, the glass keeps the photons from escaping, and guides them to its edges. There, slivers of conventional photovoltaic cells — placed vertically, rather than facing direct sunlight — gobble the photons up.
This concept was first proposed in the 1970s, but early attempts were disappointing. The dyes themselves were absorbing too much of the light, making the panels inefficient.
To prevent that, Baldo’s team mixed 1 percent of a phosphorescent dye with the fluorescent dye. The fluorescent dye absorbs photons of a specific energy. Each time a photon hits fluorescent dye molecule, the molecule swells with energy. Normally, the dye would then emit a new photon and its energy return to normal. Since the second photon has the same energy as the first, chances are it would soon be absorbed again by another dye molecule. (Just as dye molecules absorb photons coming from the sun, the dye molecules can absorb photons emitted by each other.) But ideally, to achieve greater efficiency, scientists would like the photon to be absorbed, released and then head straight to the photovoltaic panel to be turned into electricity.
But in the new design, each fluorescent molecule will be neighbored (usually less than 1 nanometer away) by a molecule of the second dye, which will sense the energetic swelling of a fluorescent molecule, and steal away its extra energy. The second dye molecule will also soon emit a photon, but of a lower energy. That “wrong” energy will enable most of the photons to fly by the fluorescent molecules without being re-absorbed.
Working with off-the-shelf materials, the MIT team was able to get 6.8 percent efficiency out of their panels — meaning to convert 6.8 percent of solar energy into electricity — but Baldo says developing new, specialized dyes for the purpose could probably double that figure. Dyes tend to absorb photons within a narrow range of wavelengths, but glass layers could be stacked, each containing a dye that picks up a different wavelength. Many experts say that low-cost panels should be at least 10 percent efficient to compete with standard photovoltaics, which can have efficiencies of more than 20 percent.
By circumventing self-absorption, the team has overcome “what seemed like a fundamental problem,” says StanfordUniversity electrical engineer Peter Peumans. “I think this work will renew interest in this type of solar collector.”
Peumans says researchers will still have some work to do, and that making the technology competitive may also require developing specialized photovoltaics for the different layers, which would pick up photons of particular wavelengths. “People will pay attention again and look back at their cabinets, and the dyes they already have,” Peumans says.







