Tracking Tumors
Looking for early signs of a therapy's success
Patience is a virtue . . . sometimes. For people with cancer, patience can be deadly–but they often have no alternative. A treatment can take months to shrink a tumor measurably, slow tumor growth, or prove itself ineffective. While doctors and patients wait on such sluggish gauges of success or failure, friends and family agonize, patients suffer side effects, and the cancer may be spreading undetected throughout the body.
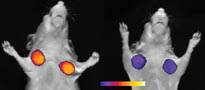
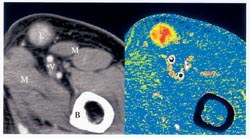
Without faster-acting measures of a treatment’s efficacy, doctors have to delay making informed recommendations about other therapy options. Earlier information would enable doctors to save more lives and alleviate suffering from side effects caused by drugs that aren’t working, says E. Edmund Kim of the University of Texas M.D. Anderson Cancer Center in Houston.
Cancer biologists and clinicians are developing tools that could make that possible. They all take advantage of new knowledge about the molecular and cellular biology of cancer.
Most oncologists today track the growth or disappearance of a tumor with X rays, computerized tomography (CT) scans, magnetic resonance imaging (MRI), or positron emission tomography (PET). Each technique uses a different approach to visualize what’s going on inside a person’s body. But when it comes to tracking cancer treatments they do essentially the same thing: produce innerbody images that doctors can use to follow the size of tumors. Even with these sophisticated scanners, it usually takes weeks or months to discern changes.
What’s needed, says Kim, are ways to detect the effect of a cancer therapy just days after treatment. Tracking cellular and molecular responses holds the most promise for filling this need, he says.
One treatment response that many researchers have been investigating is the process known as angiogenesis–the growth of new blood vessels. Because cancers don’t grow unless they get a supply of new blood and its cargo of oxygen and nutrients, molecules associated with vessel growth and images of the vessels could indicate the success of a treatment. Another new gauge for monitoring cancer therapies could take advantage of several proteins associated with the spread of cancer cells from their original tumor. Further, scientists are learning how to use chemical signals from tissues to determine whether cancer cells are dying in response to treatment.
“As we get away from measuring cancer with a ruler to measuring it on a molecular level, these methods have extraordinary promise,” says Donald A. Podoloff, a radiologist at the M.D. Anderson Cancer Center. Eventually, he says, such knowledge may help physicians select the best treatments, optimize doses, and monitor the therapeutic effects of cancer treatments much more quickly than has previously been possible.
Imaging vessel growth
In their mad rush to replicate, cancer cells stimulate the growth of new blood vessels that supply oxygen and nutrients. Researchers have already identified several compounds–such as vascular endothelial growth factor, or VEGF–that trigger angiogenesis. The new vessels themselves produce several unique cellular proteins that also might help doctors track the course of treatments.
Because tumors without a blood supply stop growing, scientists are currently working to develop cancer drugs that inhibit angiogenesis. However, says James Tatum of Virginia Commonwealth University in Richmond, “we don’t have good ways to measure their effects.” In animal models, angiogenesis inhibitors shrink tumors, an outcome that researchers can easily see using conventional imaging techniques. In people, however, the drugs typically slow the growth of a tumor but don’t reduce its size. Tatum notes also that some tumors appear to continue growing even during effective therapy because they fill with fluids as the cancer cells die.
Measurement of blood flow to a tumor is one possible indicator of the extent of angiogenesis in patients, says Marco Essig of the German Cancer Research Center in Heidelberg. He and his colleagues have paired MRI with a special contrast agent to reveal changes in blood flow to brain tumors.
The agent, after it’s injected into a person’s bloodstream, shows up clearly on the MRI. By measuring how long the agent remains in a tumor’s vicinity, the researchers can calculate how much blood is feeding the cancer. Such measurements made 6 weeks after surgery and radiation therapy were more predictive of patients’ health 3 months later than were conventional measurements of tumor size, Essig says. The Heidelberg researchers presented their findings at a meeting of the Radiological Society of North America in Chicago last November.
Other investigators at that meeting reported using X-ray-based CT and another new contrast agent to measure blood flow. In people with lymphoma, for example, changes in blood flow through tumors were evident a week after chemotherapy and preceded changes in tumor size discernable by conventional CT, says Wolfgang Römer of the University of Erlangen-Nuremberg in Germany. He suggested that alterations in a tumor’s metabolism, as detected by its consumption of a radioactively labeled sugar, might correspond to the cancer’s response to therapy.
The problem with most of the contrast agents currently available, including those being used by the German researchers, is that they quickly diffuse out of the bloodstream, blurring the image, says Francis Blankenberg, a radiologist at Stanford University. Therefore, many researchers are developing contrast agents that remain within blood vessels unless they’re abnormally leaky–a trait of new blood vessels feeding tumors, he says. Furthermore, these new contrast agents show small blood vessels clearly, so researchers can analyze a tumor’s circulatory system with precision.
Molecular markers
A different kind of imaging under development in several laboratories focuses on the biochemical signals of angiogenesis, as well as on molecules that mark the spread of cancers within the body.
To visualize tumors in mice before and after chemotherapy, researchers at M.D. Anderson Cancer Center labeled the experimental drug endostatin with a radioactive tag. This drug, which binds to molecules in new blood vessels, prevents angiogenesis and is being studied as a cancer treatment. Using a device that scans tissues for radioactivity, the team found that endostatin binding indeed correlated with new blood vessels and tumor growth, Kim reported at the November radiology meeting.
Other researchers across the country are radioactively labeling proteins involved in angiogenesis. Among the molecules under study are the alphaV-beta3 endothelial integrins. Integrins play roles in a variety of cellular functions, especially communication with molecules outside the cell. AlphaV-beta3 endothelial integrins are found specifically on recently formed blood vessels, so tracking these proteins might indicate blood vessel growth associated with cancer.
Once a tumor has become large enough to be spotted in a traditional imaging device, doctors need to know how likely that cancer is to spread, or metastasize, and whether anticancer therapies being applied are preventing metastasis. To reveal such information, researchers are looking beyond angiogenesis to other steps of cancer’s growth and spread.
A group led by Ralph Weissleder at the Center for Molecular Imaging Research at Massachusetts General Hospital in Boston and Christoph Bremer of University of Muenster in Germany has developed a contrast agent that fluoresces when it reacts with a certain enzyme involved in metastasis. The enzyme, called matrix metalloproteinase-2 or MMP-2, breaks down the tissue surrounding tumors, thereby helping cancer cells escape into the bloodstream.
“MMPs are present in all kinds of different tumors,” says Bremer, “and it is known that highly aggressive tumors with a poor clinical outcome overexpress MMPs.” At the November radiology meeting, Bremer and his colleagues reported that near-infrared fluorescence imaging reveals MMP-2 inside tumor-riddled mice as early as an hour after injection with the agent. The fluorescence correlates well with the tissues to which cancer has spread, he says. The technique could provide an effective, noninvasive way of determining a cancer’s aggressiveness, says Bremer.
In a direct approach to imaging the effectiveness of cancer therapy, researchers are trying to spot dying cells. The scientists have identified a molecular flag that the body’s immune system uses to detect cells that are stressed, dying, or dead. In normal, healthy cells, a fatty component of the cell membrane called phosphatidylserine stays inside the cell membrane. But when cells lack the energy to hold the phosphatidylserine in place, it tends to spring to the outside.
Researchers have found that a human protein called annexin binds to phosphatidylserine–if there’s access to it. Thus, annexin identifies cells that are stressed or dying, says Blankenberg. He and his colleagues have attached the protein to a radioactive compound that imaging machines can trace. The radioactively labeled annexin “will allow you to notice therapeutic success much earlier, within a day or two following the initial chemotherapy,” Blankenberg says.
In early safety and efficacy studies, people with advanced lung cancer and lymphoma in whom radioactively labeled annexin indicated cancer-cell stress or death immediately following therapy fared better than patients without those signs did. On average, the people showing the early response to treatment lived 3 to 4 months longer than the others, says Allan Green of Theseus Imaging Corp. in Boston, which hopes to market the annexin marker in the United States.
“If you don’t see any cell killing after the first exposure to chemotherapy, these patients are very unlikely to see a benefit,” says Green. “By trying to identify the effect early, we can give physicians a choice, very early on, of using other therapies.”
Imaging phosphatidylserine outside cells as an indicator of cancer-therapy success “would be a boon to therapy–though it’s still early,” says Tatum. Other teams besides Blankenberg’s are pursuing annexin as a marker for cell stress and death. Scientists at Massachusetts General Hospital’s Center for Molecular Imaging Research in Boston have bound annexin to a magnetic particle, which can then be imaged.
Beyond cancer
“Pretty much all diseases have molecular alterations at the root,” says Tatum. Cancer, heart disease, immune disorders, and inflammation are some of the diseases whose molecular underpinnings are already well known–and quite similar.
Consequently, new ways of imaging the early effects of cancer therapy could help doctors in a wide range of fields, says Kim. Monitoring angiogenesis can track not only cancer therapy but also other diseases in which blood vessel growth plays a role. For example, angiogenesis goes awry in retinopathy, a disease in which new blood vessels obscure the retina and cause blindness.
Moreover, treatment that induces angiogenesis is being investigated to help people with stroke or cardiovascular disease.
Imaging the activity of MMP-2 and other enzymes may also prove important in diagnosing or monitoring cardiovascular diseases, arthritis, and inflammation, says Ching H. Tung of Massachusetts General Hospital.
Tracking annexin, too, offers promise in diseases other than cancer. Green predicts that studies of PS using radioactively labeled annexin in people who have had heart attacks will help elucidate the extent of heart-tissue stress and death and measure the success of treatments. Tests using labeled annexin are already under way, both in people recovering from heart attacks and those undergoing heart transplants.
“Medicine is not pure science; it is the art of probability,” says Kim. “These new imaging devices will give us a better way of predicting benefit–or the lack thereof–for a therapy.” And that’s good news, he says, because for doctors battling their patient’s diseases, to be forewarned is to be forearmed.







