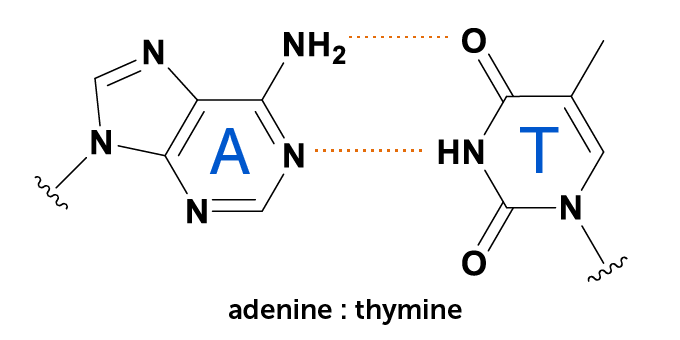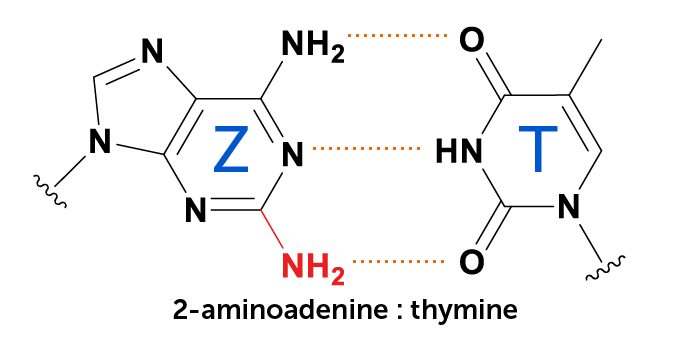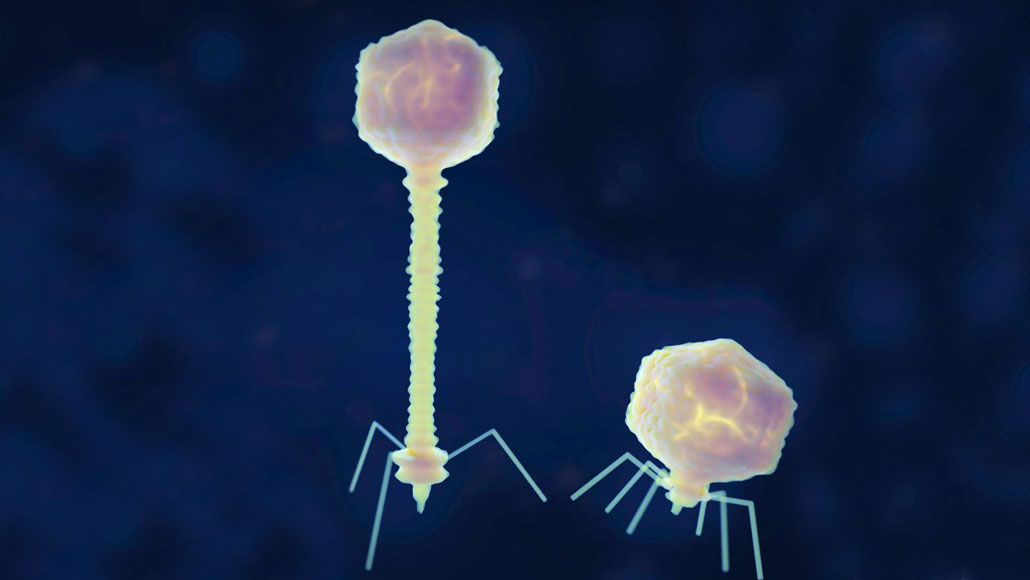
Some bacteria-infecting viruses from the Siphoviridae (left) and Podoviridae (right) families illustrated here use a different DNA letter, Z, instead of the standard A.
Science Photo Library/Alamy Stock Photo
Some bacteria-killing viruses spell out their genetic instructions in a different DNA alphabet.
More than 40 years ago, scientists in Russia reported that a type of bacteriophage called cyanophage S-2L replaces the DNA building block adenine, commonly known as A, with 2-aminoadenine, designated Z. But no one knew how the phage went from A to Z, or why.
After decades of wondering, two independent groups of scientists have discovered how the viruses make and build Z into their genetic instructions, and one reason they do it, the teams report in three studies in the April 30 Science.
The findings have implications for the origins of life on Earth, the search for life on other planets and multiple potential applications in biomedicine, synthetic biology, material sciences and computing, says Farren Isaacs, a molecular and synthetic biologist at Yale University who coauthored a commentary in the same issue of Science. “It’s a really fundamental discovery.”
In the 1990s, Philippe Marlière, a xenobiologist then at the Pasteur Institute in Paris, was “looking for examples divergent from life as we know it,” when he came across the 1977 Russian study describing the cyanophage with the unusual DNA. After getting a sample of the virus, Marlière and colleagues deciphered the phage’s complete set of genetic instructions, or genome.
In the virus’s genome, the researchers found instructions for building an enzyme, called PurZ, that could carry out the first step in making Z — also known as diaminopurine. The Pasteur Institute filed a patent on the enzyme in Marlière’s name in 2003.
With the enzyme in hand, “it became crystal clear how Z was made, but we didn’t [do] any experiments to prove that we were right,” says Marlière, now president of the European Syndicate of Synthetic Scientists and Industrialists in Berlin. The project was halted for a variety of reasons.
The researchers didn’t publish their findings until now, in part, because PurZ wasn’t the enzyme Marlière was looking for. Instead, he says he had hoped to find a different enzyme, a polymerase that would reject adenine and instead build DNA with Z in its place. “I was very, very disappointed,” he says, “because the polymerase I was craving could not be detected in that phage.”
Indeed, this phage’s polymerase isn’t what he was looking for. Marlière’s collaborator Pierre Alexandre Kaminski and colleagues found that cyanophage S-2L’s polymerase isn’t picky about using A or Z. Instead, another viral enzyme called DatZ degrades adenine building blocks, leaving the polymerase no choice but to use Z, Kaminski, a biochemist at the Pasteur Institute, and colleagues report April 23 in Nature Communications.
Periodically, Marlière searched genetic databases for other phages that have PurZ and might contain the elusive picky polymerase. Then about four years ago, he says, “I got results. Ding, ding, ding! And I didn’t get just one. I got 12. And bingo, right next door to this PurZ gene was, guess what, a polymerase gene. Aha!”
The Siphoviridae bacteriophages that infect a wide variety of bacteria all have versions of the polymerase, called DpoZ, that preferentially insert Z instead of A into the viruses’ DNA, the researchers report. Marlière has filed a patent on the enzyme.
The alternative alphabet may be used far more widely than previously thought, says Huimin Zhao, a synthetic biologist at the University of Illinois at Urbana-Champaign. He first heard about the bacteriophage that use Z-containing DNA at a dinner party a few years ago, he recalls. Not knowing that the French scientists were still working on the puzzle, he also searched databases and found 60 bacteriophages that contain PurZ, including phages from both the Siphoviridae and Podoviridae families. His team also worked out the biochemical pathway the phages use to make and incorporate Z, and found enzymes that degrade A.
Just because the phages have the enzymes, they don’t necessarily use Z in their DNA. So Zhao and colleagues in China chose a phage called SH-Ab 15497 that infects Acinetobacter bacteria, and confirmed that its DNA alphabet also has Z in place of A, his team reports.
Replacing As with Zs
Why phages would bother with the unconventional DNA was still unknown. One hypothesis is that replacing A with Z is a countermeasure against bacterial defense enzymes, known as restriction enzymes, that chop up DNA from invading phages. Such enzymes have a hard time recognizing and cutting DNA containing Z bases, Zhao and colleagues found. “The phage is trying to avoid being destroyed by the host,” he says. “This is really a protection mechanism for the phage.”
It’s also part of a never-ending arms race between phages and bacteria, says Steven Benner, a chemist and astrobiologist at the Foundation for Applied Molecular Evolution in Alachua, Fla. It’s possible that other phages that use Z or other alternative DNA bases may still be out there. “We have overlooked this life-form on Earth because our molecular tools didn’t allow us to look for it,” he says. “What these guys have done is discover an entire biosphere that was missing from our inventory.”
It’s debatable whether Z-containing phages are new forms of life (not to mention the ongoing debate about whether viruses are alive), says Floyd Romesberg, a synthetic biologist at the global pharmaceutical and biotechnology company Sanofi’s site in La Jolla, Calif. But it does open up new possibilities, he says, for what life is, was, and could become.
“Life isn’t exactly what we thought it was. Life doesn’t have to be GTAC,” he says, referring to the four letters of the standard DNA alphabet. “What it says is that life can be more diverse.”
That realization could influence the search for life on other planets (SN: 4/18/16). Scientists often assume that they should search for guanine, thymine, adenine and cytosine, the bases of DNA as we’ve known it until now. But maybe researchers should be looking for 2-aminoadenine, the Z base, instead, Benner says.
After all, Z forms three hydrogen bonds with thymine, instead of the two hydrogen bonds that hold A–T base pairs together. That makes Z–T paired DNA more stable and potentially able to stand up to hotter or harsher conditions than conventional DNA can, he says.
With the extra stability, one might wonder why all organisms on Earth don’t use Z. Stability isn’t everything, Romesberg says. DNA has to be unwound and split apart to be copied. That may be harder to do with Z–T base pairs. Z also changes how DNA curves and bends, perhaps making it harder to pack into tight spaces the way A-containing genetic material can. That might make A more attractive for other organisms.
Or perhaps it was just an accident that A came first. Once cells started using that base, too many things would have to change to completely switch to another base, says Romesberg, who has been working for years to get bacteria to incorporate exotic DNA bases (SN: 5/7/14).
Cells have a hard time swapping because there are so many different parts that would have to be changed to accommodate a new DNA base. Viruses’ stripped-down genomes are more flexible, Romesberg says: They carry around less machinery because they make the host do most of the work. Even the Z-phage do just the first step in making Z and rely on several host enzymes to finish the recipe. It’s still not known whether cellular organisms can write Z into their DNA, too.