Pesticide Disposal Goes Green
Farmers derive many benefits from pesticides. When stressed by bugs and weeds, plants and livestock don’t grow as well or as quickly. Moreover, when it comes to produce, growers know that spotted, nipped at, and deformed fruits and veggies command a lower price in the marketplace.
However, for all their benefits, pesticides can exact a high toll on the environment and the health of farm workers. Indeed, one reason these chemicals work so well at deterring bugs and weeds is that they’re usually stable, long-lasting poisons. Many are broad-spectrum agents, meaning that they can kill not only the targeted pest, but also many beneficial organisms. In sufficient doses, most can make people sick.
Over the past 2 decades, chemist Terry Collins and his colleagues at Carnegie-Mellon University (CMU) have been developing catalysts that might safely degrade dangerous stores of pesticides so that they pose less of a hazard to people and farm animals.
The CMU experiments suggest that the new catalysts may one day find use in not only degrading large quantities of pesticides in leaking storage drums—but also of treating diffuse quantities that may have tainted wastewater, groundwater, or soil.
New tests of the catalysts show that several turn common pesticides and other toxic chemicals into relatively harmless carbon dioxide and water. Related experiments indicate that the catalysts might also clean paper-mill effluent, remove sulfur from diesel fuel, and even destroy biowarfare agents. Colin Horwitz of CMU reported the team’s latest progress on the catalysts at the Society of Environmental Journalists annual meeting last month in Pittsburgh.
Oxy-cleaning
Oxidants are chemicals, such as hydrogen peroxide, that rob electrons from neighboring compounds, an event that triggers powerful chemical changes. For instance, oxidative reactions can rip apart molecules. However, on their own, many oxidants operate fairly slowly. The CMU team designed its catalysts to dramatically rev up an oxidant’s reaction rate.
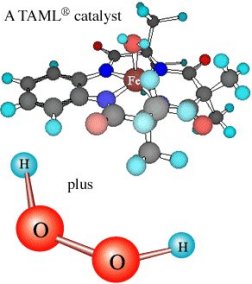
At the core of each catalyst in the new family sits an iron atom. Around it are four proteinlike constituents made from carbon, hydrogen, oxygen, and nitrogen. The CMU scientists have patented the new molecules and refer to them as “environmentally friendly,” nontoxic iron-TAMLs (for tetra-amido macrocyclic ligands). It takes only trace quantities of an iron-TAML—usually around 1 to 10 parts per million (ppm) in water—to speed oxidative reactions to commercially useful rates, Horwitz says. The catalysts in the family differ primarily in the speed at which they drive oxidizing reactions.
In some cases, a catalyst renders a molecule harmless by merely cleaving off a portion of it. In other cases, the molecular destruction is more thorough. In its most recent experiments, the CMU group has evaluated iron-TAMLs’ ability to drive a rapid and complete breakdown of fenitrothion. In the United States, this organophosphate insecticide is used primarily in household ant and roach traps. In Europe and elsewhere, however, the substance is sprayed on fruits and grain crops that occasionally are imported to the United States. In such countries, fenitrothion is also used to kill flies and other bugs that worry livestock. Wherever it exists, users of the chemical occasionally need to neutralize stores of it.
The CMU chemists demonstrated that they could destroy fenitrothion by mixing it with a water solution of trace quantities of one of the iron-TAML catalysts and hydrogen peroxide, Collins reports.
An especially attractive feature of the new catalyst, Collins says, is that once one batch of the pesticide is broken down, the water being used can be recycled to treat the next batch of the pesticide. In other words, the cleanup won’t necessarily be a problem in locations where water is at a premium.
Two years ago, these researchers reported their ability to use iron-TAMLs to destroy two chlorinated pesticide agents that pollute many water supplies and are resistant to breakdown: pentachlorophenol and 2,4,6-trichlorophenol. In the April 12, 2002 Science, the CMU scientists reported that their iron-TAML catalysts performed better against the pesticides than alternative biological and chemical treatments do. Moreover, unlike many cleanup treatments applied to chlorinated chemicals, the iron-TAML catalysts eliminated the pesticides without producing dioxins or related toxic by-products.
Catalyst wears many hats
The iron-TAMLs show promise in several areas beyond destroying stored pesticides, the scientists report. For instance, the catalysts foster the oxidative cleanup of residues in paper-mill wastewater. The coffee-colored effluent that paper mills release into rivers (see Macho Waters) can hormonally alter fish and cut off the penetration of light to underwater plants. In pilot tests with the new catalysts, Horwitz’ team showed that just 1 gram of an iron-TAML could clean the black residue from the wastewater produced in the making of 1 metric ton of paper pulp. Indeed, the chemists achieved a 78 percent reduction in effluent color—from an opaque black to a translucent yellow—and 30 percent reduction in the effluent’s chlorinated pollutants.
Another potential application, Horwitz says, is in removing sulfurous compounds from diesel fuel. These chemicals, known as dibenzothiophenes, are a particular problem for fuel manufacturers and engine designers because they can destroy the emissions-control technologies designed to prevent the formation of soot as diesel fuel burns. Sulfur in diesel exhaust also contributes to the production of acid rain.
Four years ago, the Environmental Protection Agency issued a proposed sulfur limit for diesel fuel, to go into effect in June 2006. It would restrict sulfur concentrations in the fuel to no more than 15 ppm. The CMU chemists note that their catalytic treatment of diesel fuel requires just two steps, whereas existing desulfurization processes require many more steps. In the new process, the scientists first blend the fuel with water containing a 0.5 ppm concentration of one of the catalysts. Next, they add hydrogen peroxide, warm the mix to 60°C, stir for 3 hours, and then remove the water. In small-scale tests, Collins says, the treatment removed all dibenzothiophenes.
The most dramatic application for these catalysts however, may be their prospect for decontaminating troops or gear exposed to anthrax spores. At the Society of Environmental Journalists meeting, Horwitz’ team reported experiments in which it destroyed spores of an anthrax surrogate (Bacillus atrophaeus) in under 30 minutes using an iron-TAML in a mixture of water, baking soda, and hydrogen peroxide. “A decontamination time of 15 minutes—the U.S. Army’s gold standard—is within sight,” says Horwitz.
Electron micrographs of the treated B. atrophaeus spores showed that they swelled and then began leaking their contents. Of each 100 million spores treated, only 10 viable ones remained, Horowitz reports. However, he adds, the bacterial spores used in these tests, while nontoxic, tend to be more resistant to oxidative destruction than anthrax spores are. As such, the experimental findings probably underestimate the potential effectiveness of the catalyst against the real biowarfare agent.
Moreover, says Collins, the mixture of chemicals is mild enough to leave fabrics and other materials undamaged. Indeed, he notes, in the field, troops might spray dilute solutions of the catalyst and hydrogen peroxide on people and their gear.
To clean the interior of a building—such as the Hart Senate Office Building, which sustained extensive contamination from anthrax spores in letters sent there in October 2001—decontamination crews would mist a whole building interior with separate solutions of the hydrogen peroxide and the catalyst, Collins says. “The little misty bubbles [of each solution] would then mix together,” creating a quick-acting oxidant to destroy the spores in the air and on surfaces, he says.
The CMU team’s development of iron-TAMLs is taking place at its Institute for Green Oxidation Chemistry, a center committed to finding benign chemicals for use in industry. Though none of the iron-TAML catalysts is yet on the market, CMU is in currently working out licensing arrangements for some of the chemicals.