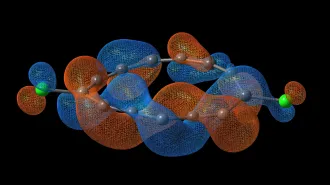
Beryllium is one of those self-loathing elements. Like helium or neon, an atom of beryllium should not partner with another, chemical theory says. But new research, published online May 21 in Science, definitively reports the nature of the beryllium-beryllium bond.
“People envisioned the beryllium atom as this sphere like a billiard ball that just bounces off another billiard ball,” says quantum chemist Rodney Bartlett of the University of Florida in Gainesville. “They’re happy with the electrons the way they are — there’s no tendency to form a bond.”
Element 4 on the periodic table, the strong, lightweight metal has two electrons in its outer shell, which could hold up to eight electrons. This arrangement means beryllium should happily team up with other elements, and it does. In nature, beryllium is a component of emeralds and also likes to bond with copper. But it should be repulsed by another beryllium atom.
Yet since the 1930s, there has been a back-and-forth between chemical theorists and experimentalists reporting that sometimes beryllium does bond to beryllium. These studies yielded wildly divergent results about the nature of the hookup. Different teams calculated different bond lengths. And there was debate over what forces made the bond possible: Some chemists said the Be2 attraction was so weak that it shouldn’t be called a bond at all.
“It is a very peculiar molecule,” says physical chemist Michael Heaven of Emory University in Atlanta, who led the new work. The Be atom is small, and the calculations that describe its electronic and molecular properties “seem like something you can do with a paper and pencil,” he says. “But it turns out to be something where you need a supercomputer.”
Heaven and his colleagues forced beryllium to bond with itself by blasting it with a stream of helium gas. Then the researchers zapped the molecule, called a dimer, with a laser that bumped an outer electron into a highly excited state. With a second laser they drove the electron back down to its original lower level. This descent released energy, producing a spectrum that was captured by the second laser. The spectrum revealed the forces acting between the atoms.
Scientists first caught beryllium in the act of bonding to itself in 1984. But the spectrographic data was incomplete and the researchers had to extrapolate what was really going on between the two atoms. “When it comes to most molecules the theories are accurate and reliable, but with this you find it’s a can of worms,” Heaven says.
If you are in a chicken coop and only glimpse the feet and tail of a bird, inferring that it is a chicken is probably a solid bet. But in beryllium’s case the equations missed the mark, incorrectly predicting the length of the bond at particular energies.
Based on the whole bird, the beryllium-beryllium bond is real, but delicate and not fully developed. The electrons of the two bound atoms swirl in a complex dance that minimizes repulsion. The new work gets measurements for bond lengths at higher energies that agree with theory. The findings also suggest that for the bond to form, the electrons actually take advantage of the next orbital out, which is typically empty. But for the electrons to keep their appropriate distances, this outer orbital hybridizes with the orbital that usually just holds beryllium’s two outer electrons.
“The work provides a unique glimpse into the process of bond formation,” Heaven says. “The situation is often encountered as reactants come together … and as they depart.”
Bartlett says the work is a reminder of how theories don’t always get everything right. This study nicely demonstrates “how dogma can lead to erroneous conclusions.”