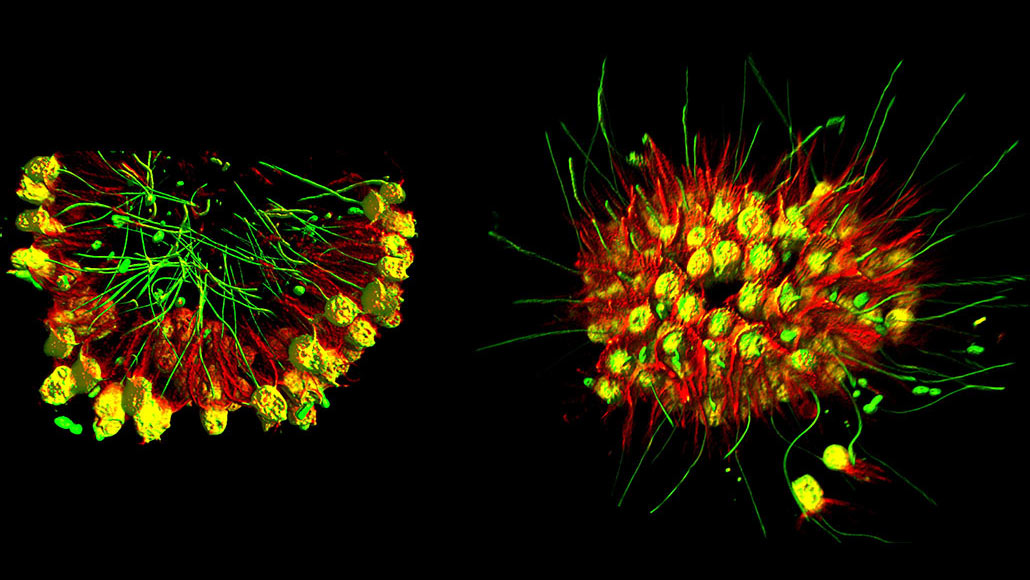
A newly discovered species of single-celled choanoflagellates forms arrays of many individuals, which can rapidly morph from feeding mode (left, shown in false color) to swimming mode (right).
Thibaut Brunet
There’s not much to a choanoflagellate. But a new species of these single-celled organisms, animals’ closest evolutionary relatives (SN: 7/29/15), could provide crucial clues to a fundamental question in biology: How did solitary cells band together long ago to form multicellular coalitions capable of moving, hunting and hiding?
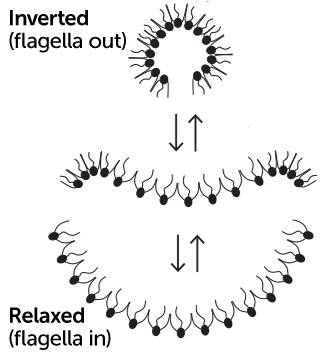
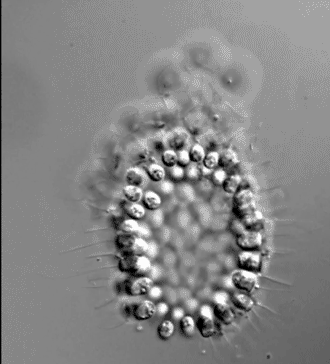
Most choanoflagellates live simple, solitary lives. So when cell biologist Nicole King, a Howard Hughes Medical Institute investigator at the University of California, Berkeley and her colleagues discovered hundreds of these organisms locked together in a sample taken from a splash pool along the coast of the Caribbean island of Curaçao, they were surprised. The cells formed a concave sheet, with their tail-like flagella extending from the cupped side.
What happened next stunned the scientists. In unison, the organisms making up the sheet inverted into a ball-like shape, tiny flagella flailing outward like tiny oars, allowing the organisms to swim much more swiftly. Accordingly, the team dubbed the new species Choanoeca flexa.
“It was this crazy behavior unlike anything we’d ever heard of in choanoflagellates,” King says, “We just had to figure out how they pulled it off.”
This collective behavior emerges from the simple actions of cells responding to changes in light, King and her colleagues report in the Oct. 18 Science. The researchers suggest that the new species could offer clues to how a key step in animal evolution happened. “Plus, it’s just a really cool phenomenon,” King says.
William Ratcliff, an evolutionary biologist at Georgia Tech in Atlanta who wasn’t involved in the study, agrees. While it’s impossible to go back in time to observe how the common ancestor of animals and choanoflagellates evolved into more complicated multicellular creatures, he says, “this study breaks down this huge jump and shows how single cells can adapt and become more complex at the multicellular level.”
To get a clearer picture of C. flexa, King’s team brought the organisms back to the lab. Each individual resembles a sort of smooshed sphere. From one end, many tiny, tentacle-like protrusions form a collar that’s accented with a single, longer flagellum that extends beyond the collar.
Individual choanoflagellates join together by touching these collars. In the concave form, the flagella all point inward, “which aids feeding on bacteria,” King says. When the organisms flip into a more of a sphere, the flagella all point outward, becoming hundreds of tiny paddles that help with swimming.
Exactly what triggered C. flexa’s transformation remained a mystery until the researchers noticed that the flipping stopped when the organisms were exposed to a microscope’s light for too long. On a whim, King tried turning off the lights then turning them back on. In the dark, C. flexa inverted into a ball shape. “And then we did it again, and did it again, and did it again, and every time we changed the illumination, they flipped.”
The researchers haven’t fleshed out the full mechanism, but they’ve confirmed that a light-sensitive protein known as rhodopsin plays a role. And the collective behavior doesn’t seem to be the result of complicated communication among the cells. Rather, it stems from a simple, musclelike tightening or loosening of each choanoflagellate’s collar appendages. In sheet mode, the collars of all cells are tighter, pulling the cells into a slightly cupped shape. When the light changes, each cell’s collar widens, collectively forcing the sheet to invert into a sphere.
This change in a single choanoflagellate wouldn’t amount to much, Ratcliff says. But together, this simple individual action adds up to produce a whole new behavior — swimming or staying put to feed. “It’s a beautiful example of how simple groups of cells gain these emergent multicellular traits,” he says.
King isn’t sure why changes in light trigger this response. But she notes that a consequence of swimming faster in darkness and staying put in light is that C. flexa tends to move toward well-lit areas that might have more food. Individual cells can’t effectively swim toward light; groups of C. flexa can.
The importance of this kind of shape-shifting extends far beyond choanoflagellates, King says. Key components of animal development involve the folding of tissues as an embryo develops. “Our study shows that the basic cellular machinery necessary for this kind of folding predates the origin of animals,” she says.