Moderna’s COVID-19 vaccine stimulates an immune response in people
Early results suggest a coronavirus vaccine works, but more human trials are needed
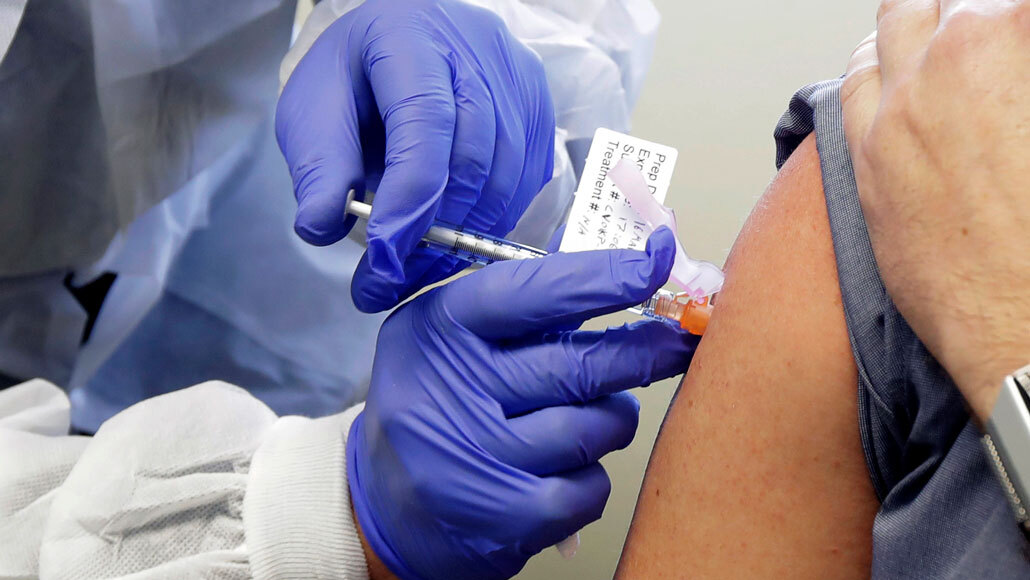
On March 16, Neal Browning was injected with an experimental vaccine (shown). Now early results from the trial show that the vaccine triggers production of antibodies against the coronavirus.
AP Photo/Ted S. Warren
An experimental vaccine may help protect against a coronavirus infection, preliminary results from people and mice suggest.
One or two doses of an mRNA vaccine prod people’s bodies to make as many or more antibodies against the coronavirus as are made by people who have recovered from COVID-19, researchers from Moderna, Inc., announced May 18.
Moderna, based in Cambridge, Mass., and the U.S. National Institute of Allergy and Infectious Diseases in Bethesda, Md., worked together to develop the vaccine, known as mRNA-1273 (SN: 2/21/20).
Their approach uses messenger RNA, or mRNA, a genetic molecule that cellular machinery reads in order to build proteins. In this case, the mRNA contains instructions for building the coronavirus’ spike protein, which helps the virus enter human cells. The vaccine induces human cells to make the spike protein. The immune system then makes antibodies to latch onto the spike proteins. Should a vaccinated person encounter the virus later, those vaccine-stimulated antibodies may prevent the virus from infecting healthy cells.
Mice vaccinated with these mRNAs were protected against lung infection when researchers later exposed the rodents to SARS-CoV-2, the coronavirus that causes COVID-19, the researchers also report. None of the data has been reviewed by independent scientists, and has not yet been made publicly available.

Trustworthy journalism comes at a price.
Scientists and journalists share a core belief in questioning, observing and verifying to reach the truth. Science News reports on crucial research and discovery across science disciplines. We need your financial support to make it happen – every contribution makes a difference.
The new data “give us confidence that mRNA-1273 has a high probability of providing protection against COVID-19 disease in humans,” said Stéphane Bancel, chief executive officer at Moderna.
Researchers in Seattle and Atlanta began injecting human volunteers with the experimental vaccine in March (SN: 4/10/20). This first phase of testing was designed to determine safety, help researchers determine what dose to use, and to measure people’s immune response.
The study tested three doses: 25 micrograms, 100 micrograms and 250 micrograms. People in each dosage group got an initial injection, followed a month later by a booster shot. Although 45 people participated in the study (15 in each group), antibody results for only the first four people in the 25-microgram and 100-microgram groups are available. Some preliminary results for people in the 250-microgram group were also reported.
Levels of antibodies called neutralizing antibodies, which can block the coronavirus from infecting human cells, measured two weeks after the booster shot were equivalent between the 25-microgram vaccine group and in people who had recovered from a COVID-19 infection. Levels of neutralizing antibodies in the blood of the 100-microgram group were even higher than in people who had recovered from the disease.
“These data are extremely promising, but they are in eight people,” says Brianne Barker, an immunologist at Drew University in Madison, N.J. “I agree with Moderna and NIAID that they should keep going” with the vaccine, she says. “But I don’t know that we fully know yet that this is going to be a home run.”
Other scientists also say that the available data are too minimal to draw firm conclusions. Neutralizing antibody levels in blood can vary widely, says Paul Bieniasz, a virologist at the Rockefeller University in New York City. “So when Moderna says ‘levels matching or exceeding the level found in [recovered people],’ that’s almost meaningless.” And no one yet knows what level of antibody production is necessary for protection in people, nor how long such protection might last.
The study measured only one type of immunity, that produced by neutralizing antibodies. The researchers didn’t measure whether other immune cells called T cells also rev up virus defenses in response to the vaccine, Barker says. Such T cells may protect people from developing severe cases of COVID-19 (SN: 5/15/20).
One person in the 100-microgram group developed redness at the injection site, and three people in the 250-microgram group developed some severe flulike symptoms, including muscle aches, headache and fatigue, after the second injection. Those symptoms are probably indications that the immune system is responding to the vaccine, said Tal Zaks, Moderna’s chief medical officer, during a May 18 briefing with reporters and investors.
But those symptoms could be cause for concern. The injection site redness was classified as severe, or grade 3. That’s a red spot 10 centimeters in diameter, says Mark Slifka, a viral immunologist at Oregon Health & Science University in Portland. The next step is death of the tissue. Milder side effects are typically common with vaccines, “but if there are a consistent number of grade 3s, that’s a concern for a vaccine you want to use broadly.”
The company has already gotten permission from the U.S. Food and Drug Administration to go ahead with the second phase of human safety tests. The company will eliminate the 250-microgram-dose arm and add a 50-microgram group. Lower doses may offer equal protection against the virus and stretch limited supplies of the vaccine, Zaks said.
The initial tests were in people 18 to 55 years old. The next phase of testing will also include people 56 to 70 years old, and an over-70 group.
A much larger third phase of testing, which would continue safety testing and measure antibody responses, will also determine whether the antibodies actually protect against infection. That phase may begin as early as July.







