Ebola vaccine protects people in West Africa
In Guinea trial, zero cases of virus occurred in people potentially exposed who received immediate shots
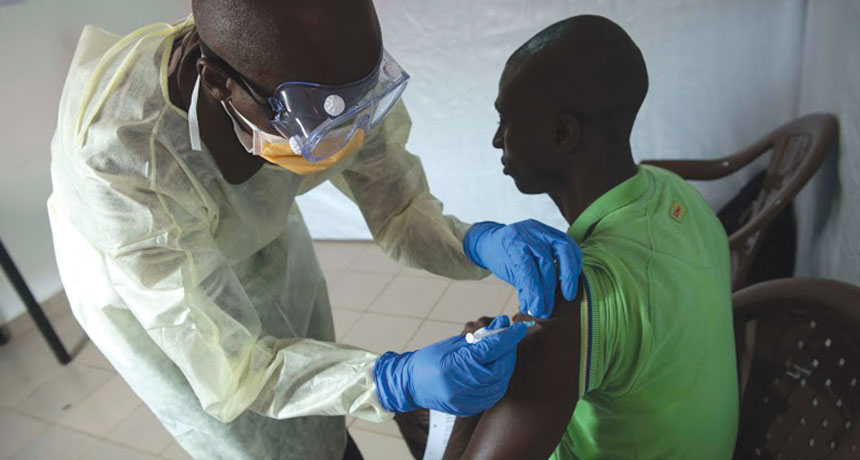
PROTECTIVE JAB A study of more than 7,000 people in Guinea shows that an Ebola vaccine guards against the lethal virus.
©Yann Libessart/MSF
The first large test of an Ebola vaccine in the field shows strong protection against the lethal virus. With the epidemic in West Africa now in retreat, the shot might hasten disease elimination in Guinea, which still has cases cropping up.
“This is a huge advance in the Ebola field,” says Thomas Geisbert, an immunologist at the University of Texas Medical Branch at Galveston. “It’s been 10 years, but we’re finally getting it into people.” In 2005, Geisbert and virologist Heinz Feldmann of the National Institute of Allergy and Infectious Diseases developed the vaccine when they were working for the U.S. and Canadian governments, respectively (SN: 7/16/05, p. 45). The vaccine uses a live virus called vesicular stomatitis that causes only mild infection in humans. It contains an Ebola glycoprotein that cannot cause disease but does trigger immunity.
An international team of researchers tested the vaccine in Guinea this year using a “ring-vaccination” strategy. When an Ebola case was diagnosed, the patient’s close contacts and a cluster of people living in the area were randomly assigned to one of two groups: People in one group received the vaccine’s single shot immediately; others after a 21-day delay. The scientists established 90 such clusters, with 7,651 adults getting vaccinated, about half in each group.
Among people who got the delayed vaccination, 16 contracted Ebola. In the prompt-vaccination clusters, none did, the researchers report July 31 in the Lancet. The scientists counted only Ebola cases that showed up 10 days or more after people entered the study, to rule out preexisting cases.
Based on these results, the still-experimental vaccine will continue to be used throughout the country, as requested by the Guinean government. But it will be given without delay in clusters around new infections, the researchers report. Scientists will continue to collect data on vaccine effectiveness and safety.
The trial may also have had an impact on the epidemic. Public health measures have been the main weapon against the West African outbreak, but they haven’t fully stamped it out. The vaccine, dispensed from April 1 through July 20, has contributed to the fight in Guinea, says study coauthor John-Arne Røttingen, an infectious disease doctor and director of the Division of Infectious Disease Control at the Norwegian Institute of Public Health in Oslo. “It really is a countermeasure that should help get us to zero.”
The trial’s approach might prove useful in future outbreaks, says Mark Feinberg, an infectious disease doctor and chief of public health and science at Merck in West Point, Pa., the pharmaceutical company that manufactures the vaccine. “Before this outbreak, there wasn’t a path successfully navigated for how to evaluate the efficacy of a vaccine in the middle of an outbreak,” he says.
At last count, about 20 new cases per week have been cropping up in West Africa. But in the week ending on July 26, only four arose in Guinea and three in Sierra Leone, the World Health Organization reports. In all, there have been 27,784 cases of Ebola and 11,294 deaths since the outbreak began in the region some 18 months ago.
A lot more work is required before the vaccine can get regulatory approval, Feinberg says. Researchers need to ascertain how well it works in specific groups such as children and the elderly, and regulators need to establish that batch after batch of the vaccine are produced uniformly and are safe for use in the field, he says.
Ideally, in the initial stages of an epidemic, the vaccine would go to people at the front lines including health workers, Geisbert says. “In the future, if you have an outbreak, I could see a multipronged approach.” In addition to standard public health measures, he says, “you could vaccinate health care workers before they get there — before they’re sent into the hot zone.” If approved, the vaccine could ultimately “be used in the context of fighting outbreaks,” he says.
Editor’s Note: This story was updated August 12, 2015, to correct John-Arne Røttingen’s title. He’s the director of the Division of Infectious Disease Control at the Norwegian Institute of Public Health, not director of the Institute.