Experimental vaccines protect children from hand, foot and mouth disease
Shots prevent cases resulting from enterovirus 71
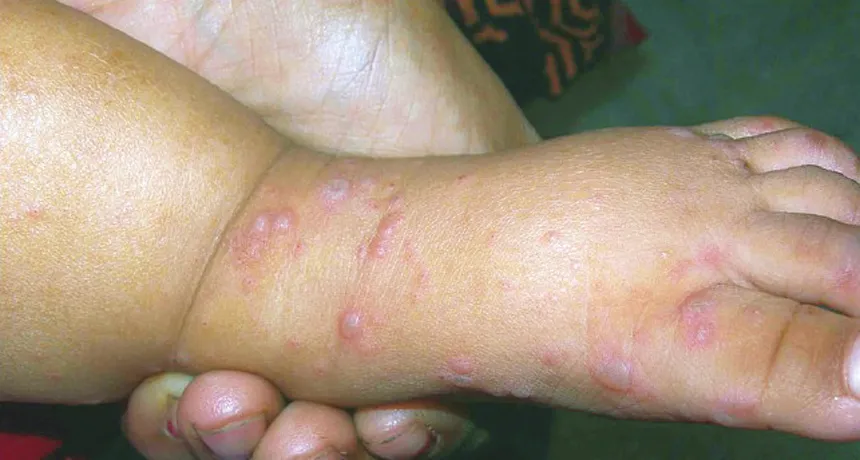
HELP ON THE WAY New vaccines against hand, foot and mouth disease that are testing well in China might slow the scourge, which has hit parts of Asia especially hard.
N. Sarma et al/Indian J. Dermatol. 2009/Open-I, NLM/NIH
New vaccines designed to fend off a leading cause of hand, foot and mouth disease have proved highly effective. Two studies involving thousands of children in China depict the vaccines slamming the door on cases arising from enterovirus 71, a common cause.
Hand, foot and mouth disease typically strikes children and is spread by unsanitary conditions. Not to be confused with the livestock illness called hoof-and-mouth or foot-and-mouth disease, the human disease shows up as fever, sore throat and blisters on the hands, feet and mouth. In rare cases, complications arise such as fluid accumulation in the lungs, paralysis or brain inflammation. Some cases are fatal.
The vaccine findings, which appear in the Feb. 27 New England Journal of Medicine, offer good news for the Asia-Pacific region where hand, foot and mouth disease has become a major health problem in recent decades. China had nearly 490,000 cases reported in 2008 and more than 1 million in each of 2009 and 2010, according to the Chinese Center for Disease Control and Prevention. Malaysia, Singapore, Taiwan, Vietnam and other countries have had outbreaks as well.
“In parts of Asia, it goes like a wildfire through communities every two or three years. A whole country may grind to a halt for a few weeks,” says Tom Solomon, a neurologist at England’s University of Liverpool who has studied enterovirus 71 in Malaysia. “Vaccination is a major step forward,” he says.
The two experimental vaccines tested use slightly different inactivated forms of enterovirus 71. Both trigger the immune system to build an army of antibodies against the real pathogen.
In one trial, 12,000 children between 6 months and 6 years of age received either two shots of the vaccine four weeks apart or two placebo shots. The vaccine was highly effective. While 151 children who got the placebo shots developed illness caused by enterovirus 71 over the next 11 months, only four in the vaccinated group came down with it. The follow-up spanned two peak epidemic seasons in China.
In the other study, 10,007 children who were 6 months to 3 years old similarly received placebo or vaccination shots. Over a year, that vaccine also showed strong protection: 106 of the children who got the placebo, but only 13 of the vaccinated kids, developed disease from enterovirus 71.
The second research team also tracked severe illness and found that 24 children in the placebo group ended up in the hospital while none of the vaccinated children did.
“Our study indicates that infants and young children can be protected against enterovirus 71–associated hand, foot and mouth disease,” says Nan Wang, a coauthor of the study of 10,007 children. She is a vaccine researcher and vice president at Sinovac Biotech in Beijing, which makes the vaccine used in that trial and provided support for it. While enterovirus 71 doesn’t cause all cases of the disease in China, she says, it accounts for a lot of them. From 2008 to 2012, she says, 45 percent of mild cases, 80 percent of severe cases and 90 percent of fatal cases resulted from enterovirus 71 infections.
Another virus called coxsackievirus has recently caused outbreaks of hand, foot and mouth disease in China, Singapore and Hong Kong. Solomon notes that most outbreaks of the illness in Europe and the United States stem from coxsackievirus, which is less likely to cause severe disease. The experimental vaccines aimed at enterovirus 71 don’t prevent infection by coxsackievirus, Wang says.







