New computer calculations have revealed that a century-long assumption in chemistry is wrong.
At the crux of the matter is the movement of the hydroxide ion, OH–, in water. Hydroxide ions and protons, H+, are important players in the acid-base chemistry vital to many important chemical processes, including photosynthesis, the pumping of protons across biological membranes, and regulation of acidity in the body.
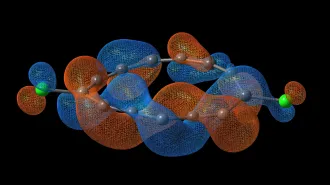
Protons’ movement through water is well understood, and scientists had long believed that the motion of hydroxide ions could be inferred from it. Researchers know that a water molecule containing an extra proton forms weak bonds with three surrounding water molecules. As this positively charged ion–which is called hydronium, or H3O+–moves through water, those bonds break and shift. They temporarily form new structures until the ion is once again bonded weakly to three water molecules in a nearby location.
Since the hydroxide ion looks like a water molecule missing a proton, chemists had assumed it behaved in a corresponding way, but one that takes the ion’s negative charge into account.
Wrong, says Mark E. Tuckerman of New York University. In the June 27 Nature, Tuckerman and his colleagues in Europe report that a hydroxide ion moving through water weakly bonds to four, not three, water molecules. The hydroxide ion also forms more-complicated intermediate structures in water than the hydronium ion does.
Moreover, certain quantum mechanical effects that influence a hydroxide ion’s movement have significantly less effect on a hydronium ion in water, says Tuckerman.
On a fundamental level, this understanding of the minutiae of ions’ movement will update textbooks, says Tuckerman. On a more practical note, he adds, it could inform fuel cell designers how the materials they use behave on a microscopic level.
“The present work is an interesting advance,” comments Arieh Warshel of the University of Southern California in Los Angeles. However, he says, the researchers’ calculations consider only 32 water molecules around each ion and don’t include an energy barrier that water molecules must overcome as they change their structure and permit passage of the ions.
These limitations of the calculation method might not significantly alter the findings of Tuckerman’s group, Warshel points out.
According to Tuckerman, the updated picture of water probably wouldn’t have changed much had the calculations included more water molecules, but it would have taken a lot more supercomputer time. The 32-molecule calculations, he notes, took more than a year.