
MARKED FOR DESTRUCTION The malaria-carrying mosquito Anopheles gambiae’s days may be numbered. Scientists have devised a gene drive that bottomed out the mosquito’s populations in lab tests.
James D. Gathany/CDC
A new gene drive may push a species of malaria-carrying mosquito to extinction.
In a small-scale laboratory study, the genetic engineering tool caused Anopheles gambiae mosquitoes to stop producing offspring in eight to 12 generations, researchers report September 24 in Nature Biotechnology. If the finding holds up in larger studies, the gene drive could be the first capable of wiping out a disease-carrying mosquito species.
“This is a great day,” says James Bull, an evolutionary biologist at the University of Texas at Austin who was not involved in the study. “Here we are with a technology that could radically change public health for the whole world.”
Gene drives use the molecular scissors known as CRISPR/Cas9 to copy and paste themselves into an organism’s DNA at precise locations. They’re designed to break the rules of inheritance, quickly spreading a genetic tweak to all offspring.
The new gene drive disrupts a mosquito gene called doublesex. Female mosquitoes that inherit two copies of the disrupted gene develop like males and are unable to bite or lay eggs. Males and females that inherit only one copy of the disrupted gene develop normally and are fertile.
Altered development
Female Anopheles gambiae mosquito’s development was altered by a gene drive that disrupts the doublesex gene. Females that inherited two copies of the gene drive (bottom right) developed antenna (red arrow) and claspers (blue arrow and enlarged images) similar to males. The females’ mouth parts also changed, preventing them from biting to get a blood meal. These alterations made the females unable to lay eggs.

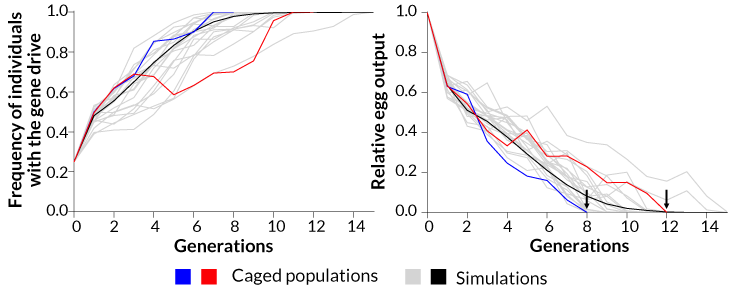
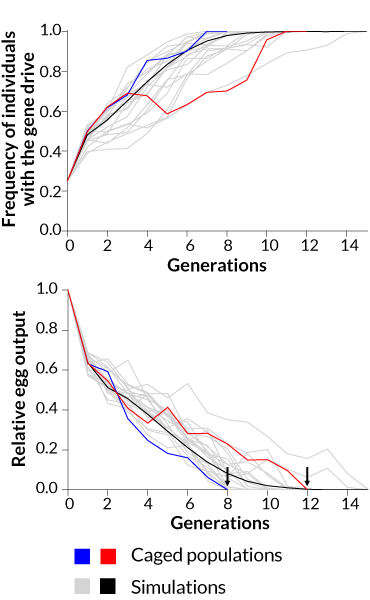
In each of two cages, researchers mixed 300 female and 150 male normal A. gambiae mosquitoes with 150 males carrying the gene drive. Each generation, 95 percent to more than 99 percent of offspring inherited the gene drive. Normally, only 50 percent of offspring inherit a gene.
Within seven generations, all of the mosquitoes in one cage carried the gene drive. No eggs were produced in the next generation, and the population died out. In the other cage, it took 11 generations for the gene drive to spread to all of the mosquitoes and crash the population.
Other gene drive studies have done computer simulations to predict how long it would take for the drives to spread through a population. This is the first time the approach has succeeded in actual mosquitoes.
Previous versions of gene drives also have been passed to offspring at high rates (SN: 12/12/15, p. 16). But those experiments were plagued by mutations that destroy the cutting site for CRISPR/Cas9, making the mosquitoes that carry the mutation resistant to the drive.
A few mosquitoes in the new study also developed mutations, but “no resistance was observed,” says study coauthor Andrea Crisanti, a medical geneticist at Imperial College London. That’s because those mutations broke the doublesex gene, producing sterile females that couldn’t pass the mutations on to the next generation.
Path to extinction
As more mosquitoes within two caged populations (red and blue lines) inherited a gene drive, the numbers of mosquitoes plummeted, finally producing no offspring after eight and 12 generations. The trend observed in the lab aligned with computer simulated predictions (grey and black lines).
All insects have some version of doublesex. “We believe that this gene may represent an Achilles heel for developing new pest control measures,” Crisanti says.
The tool raises the prospect of intentionally causing the extinction of a species. A. gambiae is the main mosquito that spreads malaria in Africa. Malaria kills more than 400,000 people each year worldwide, according to the World Health Organization.
“If you have a technology that could eradicate that [mosquito], it would be unethical not to use it,” says Omar Akbari, a geneticist at the University of California, San Diego, who was not involved in the work. But Akbari thinks it is unlikely that the gene drive would work as well in the wild, because resistance is bound to pop up at some point.
No one knows the ecological consequences of removing mosquitoes, either, or if the gene drive could be passed to other species. What if a “James Bond–style villain” used a similar gene drive to attack honeybees or other beneficial insects, says population geneticist Philipp Messer of Cornell University. “Humans will always come up with ways to abuse [technology], and in this case, it’s just so easy. That’s what worries me.”







