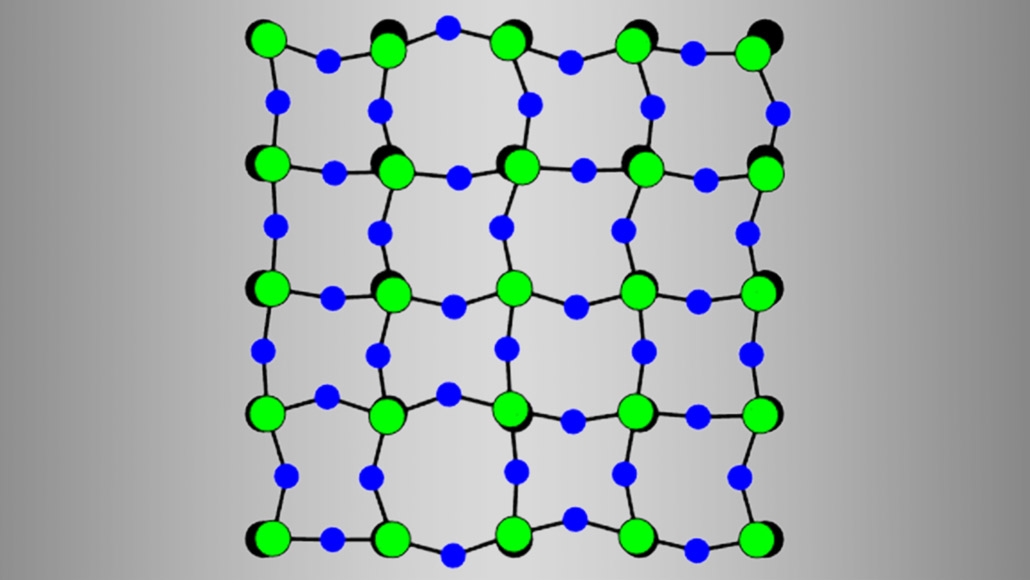
In a scandium fluoride crystal, the distance between atoms of scandium (illustrated in green above) and fluorine (blue) stays almost the same at higher temperatures. But fluorine atoms can move closer and farther apart from each other, causing the crystal to shrink.
Brookhaven National Laboratory