Mom’s immune system and microbiome may help predict premature birth
How to find and interpret markers of early labor
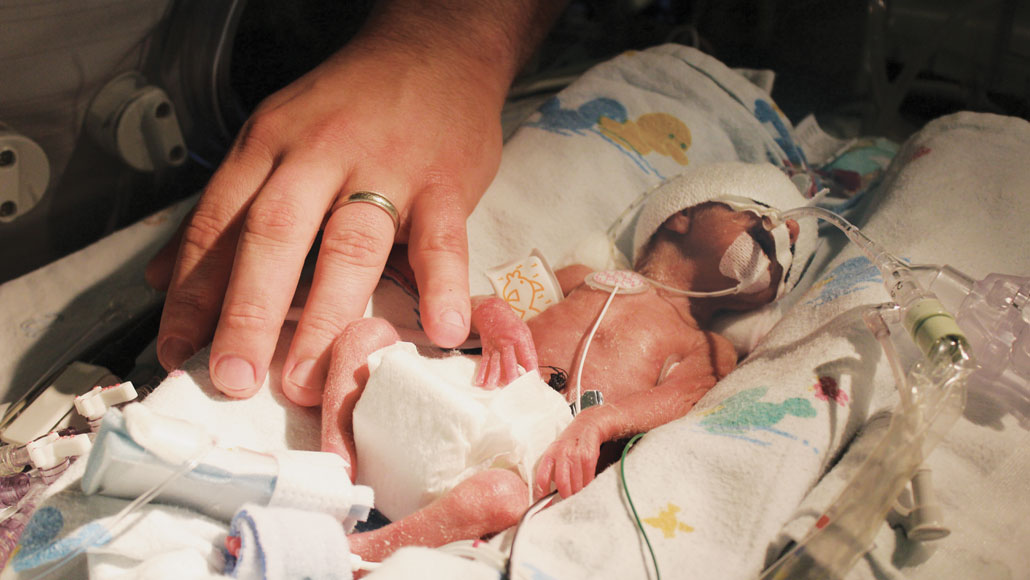
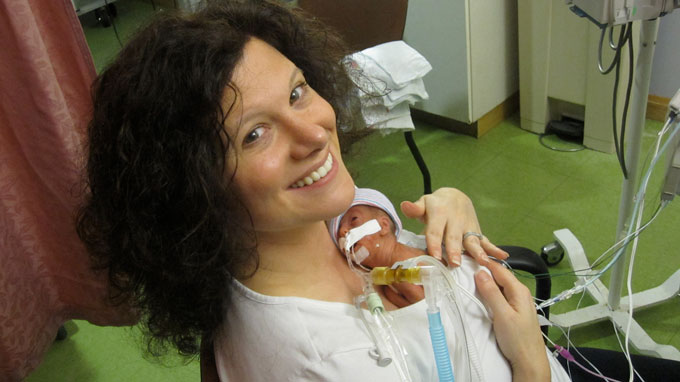
Joy Degl (pictured) was born early and weighed just one pound, four ounces. She spent 121 days in the NICU, fighting infections, eye disease and breathing problems.
Courtesy of J. Degl
Every Monday, Jennifer Degl leads a group through the halls of the neonatal intensive care unit at Maria Fareri Children’s Hospital in Valhalla, N.Y. The volunteers offer support to the parents of babies born early and struggling to survive.
Seven years ago, Degl, a high school science teacher in Putnam County, was one of those anxious parents. Her daughter, Joy, was born at 23 weeks gestation, weighing just over a pound. The baby spent her first four months in that NICU. Degl wasn’t allowed to hold Joy or change her diaper for a month.
Although already a mom to three boys, Degl was completely unprepared for the experience.
“People call [the NICU] a roller coaster for a reason,” she says. Joy would gain half an ounce and do well enough for Degl to start the 45-minute drive home to spend time with her sons, only to be called back because the baby was having trouble breathing and needed a breathing tube. “There is no smooth NICU ride,” Degl says.
Like Joy, roughly 10 percent of children worldwide — an estimated 15 million babies — are born prematurely, or before 37 weeks gestation, each year. In developed countries, surviving an early birth has become more likely, thanks to the availability of intensive medical care. More than 98 percent of U.S. preemies survive infancy, according to a study published in the American Journal of Obstetrics and Gynecology in 2016, though as many as 44 percent of the youngest preemies don’t make it. Survival is least likely in nations with the fewest resources. Worldwide, complications associated with preterm birth are the leading cause of death in children younger than 5 years old.
But survival is just step one. Many preemies face breathing problems, infections and other complications that can cause issues well after infancy. Children born prematurely can experience developmental delays and have a higher risk of learning disorders such as attention-deficit/hyperactivity disorder. Many require physical, speech and other types of therapy well into childhood. In the United States, health issues related to prematurity collectively cost more than $26 billion a year.
Joy has needed physical therapy, feeding therapy and occupational therapy. She still receives speech therapy twice a week, and because of scar tissue in her lungs from the NICU ventilators, she wheezes when she exerts herself. A simple cold is dangerous — she’s had pneumonia eight times.
For decades, researchers and clinicians have sought ways to predict and prevent preterm birth with little progress to show for it. “It’s extremely frustrating,” says neonatologist Sylvain Chemtob of Centre Hospitalier Universitaire Sainte-Justine in Montreal, who has worked in the field for 35 years. The best predictor of preterm labor is whether a woman has experienced it before. Other risk factors include carrying multiples, having a short cervix and medical conditions such as diabetes or high blood pressure.
But about half of preterm deliveries involve no known risk factors at all. “There’s plenty of room to improve,” says Brice Gaudilliere, a physician-scientist at Stanford University.
Gaudilliere and others are looking to the human immune system for clues. “The immune system is exquisitely sensitive to all sorts of environmental changes,” he says, including the mother’s nutrition and stress. The immune system could be the biological common denominator for the many known and suspected factors that contribute to preterm labor.

Immune-related genes and proteins involved in inflammation have been linked to preterm birth for decades, but such links have not resulted in predictive tests or treatments. “It’s one thing to say that the relationship between inflammation and preterm birth is known,” says Nima Aghaeepour, a machine learning scientist at Stanford. “It’s another thing to ask what are we going to do with this information.”
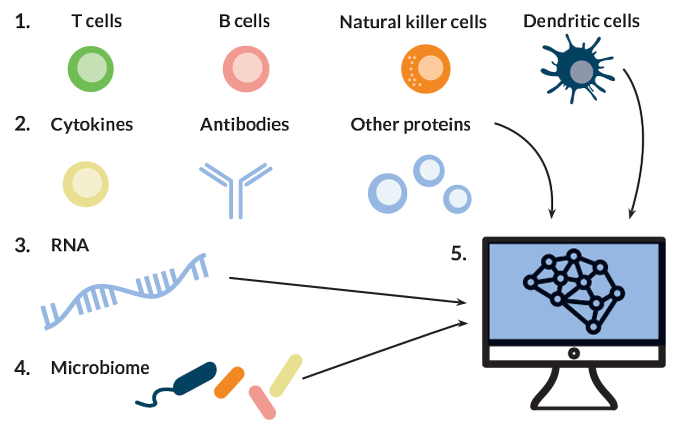
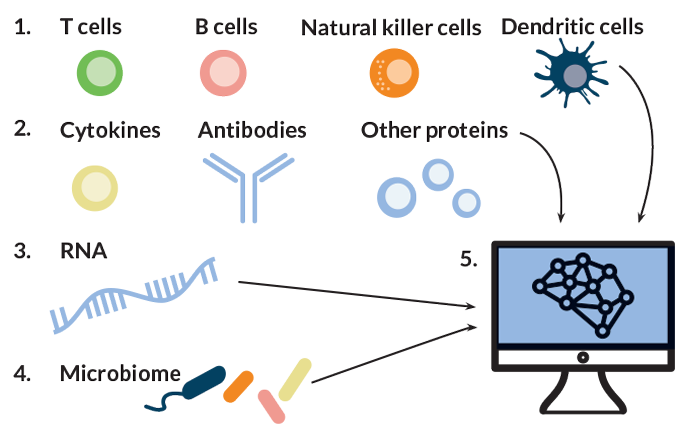
To bridge this gap, Gaudilliere and Aghaeepour are collaborating to examine the immune system as a whole — dozens of cell types, hundreds of molecules and thousands of genes. These researchers and others are using this systems immunology approach to find ways to predict a woman’s risk of premature labor based on a small sample of her blood, and then reduce that risk.
An inflammatory process
As soon as a woman becomes pregnant, her immune system changes. Her body releases chemicals that keep immune cells from attacking the embryo’s cells as foreign invaders. Once the early ball of cells implants into the wall of the uterus, a thick layer of tissue called the decidua starts to form between mother and embryo. For the rest of the pregnancy, molecules released by the placenta and uterus, as well as anti-inflammatory immune cells such as regulatory T cells, keep the immune system at bay.
When the pregnancy reaches full term, at 37 to 40 weeks, the uterus somehow switches out of this immune suppression, says Sam Mesiano, a reproductive biologist at Case Western Reserve University in Cleveland. Immune cells flood the area and set off a chain reaction that ultimately triggers the uterus to contract. Inflammation also causes cells to release enzymes that dissolve the membranes surrounding the fetus, which break and release amniotic fluid. “All these things get switched on by this inflammatory process,” Mesiano says. “That’s what we want to happen.” But not before 37 weeks.
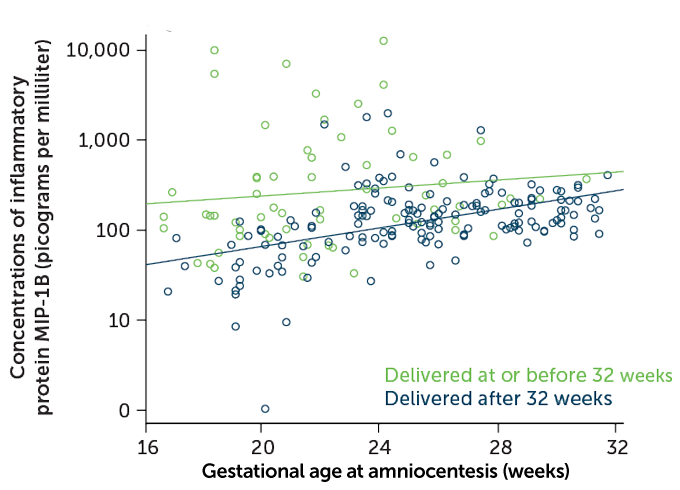
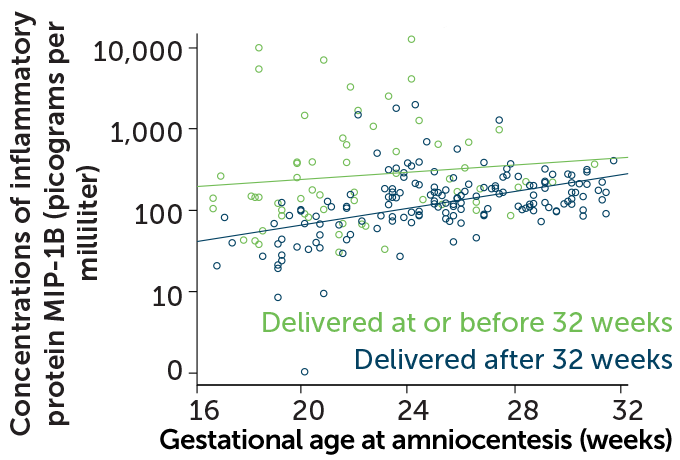
Some of the signs of inflammation linked to preterm birth differ from those found during full-term birth, says Nardhy Gomez-Lopez, a reproductive immunologist at Wayne State University in Detroit. For example, in 2017, she and colleagues reported in the American Journal of Reproductive Immunology that some proteins involved in inflammation, called cytokines, were present at higher than normal levels in amniotic fluid from a subset of women who delivered preterm. The earlier the women delivered their babies, the higher the cytokine levels. Infections, which are present in at least a quarter of preterm births, could be the cause, but inflammation and cytokine levels were also elevated when no infection was found.
Obstetricians sometimes measure cytokine levels in amniotic fluid, but only when preterm labor has already begun and an infection is suspected. Gomez-Lopez says researchers have to back up and look for reliable immune markers that are detectable in blood and tie them to the changes seen in the amniotic fluid. “We think that by studying the systemic [immune] response in the mom, we can predict these changes way earlier,” she says.
Cast a wide net
Part of the problem with developing a predictive test is that preterm labor isn’t just one condition. Thirty years ago, preterm labor was viewed simply as regular labor that happened early, says perinatologist Roberto Romero at Wayne State, who directs the perinatology research branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or NICHD. Although scientists now recognize that the biology of preterm labor is distinct, they still have to grapple with the reality that it varies depending on the underlying cause. The cells and molecules active during preterm labor brought on by infection, for example, are different from those active in labor brought on by a drop in the hormone progesterone.
Because not all causes of preterm labor are known, it is hard to find biological markers for each case, Romero says. A system-wide analysis can help because researchers don’t have to know in advance which gene or protein to focus on, he says. By examining, for instance, all the active genes in a woman’s white blood cells, or all the proteins present in a blood sample, researchers can flag differences in the immune systems of women who deliver prematurely versus those who deliver full-term.
Wayne State and NICHD recently released gene activity data from the whole blood of 150 Detroit women, 71 of whom delivered preterm, and encouraged researchers to use the data to find predictors of preterm labor, as part of a crowdsourcing collaboration called the DREAM challenge. The challenge is expected to be completed in January 2020.
Aghaeepour and Gaudilliere are taking the systems immunology approach a step further, beyond measuring gene activity. Their teams are also collecting data on the cells that contain those active genes, tracking fluctuations in the numbers of those cells, studying which molecules are produced, how active each cell type is and how those changes affect other immune factors. Casting a wide net is important, Gaudilliere says, because if one type of immune cell is responding to something, other types are probably also involved. “It makes little sense to focus on one or the other cell type,” he says.
When Gaudilliere joined the March of Dimes Prematurity Research Center at Stanford in 2015, he quickly realized how little was known about immune cells throughout pregnancy and labor. “From a basic physiological understanding of even normal pregnancy, we’re just scratching the surface, especially in the domain of immunology,” Gaudilliere says. One of the first things he and Aghaeepour, who joined in 2017, did was set up a study to establish what the immune system looks like throughout full-term pregnancy. It was part of their homework, Gaudilliere says.
The two recruited 21 women to donate three blood samples over the course of pregnancy and analyzed close to 1,000 features of the women’s immune systems at each time point. Features included measurements of 24 types of immune cells, the levels of immune-related molecules present in each cell type and the cells’ ability to respond to stimuli in laboratory experiments.
Putting it all together, the team developed an “immune clock” of pregnancy — a mathematical model that links the many immune parameters with how far along a pregnancy is. The model, reported in 2017 in Science Immunology, accurately estimated the gestational ages of a new set of 10 pregnancies. Now the team is studying whether women who go on to deliver prematurely diverge from the immune clock. With help from collaborators at the University of Chicago, the group is refining the algorithm by incorporating immune changes found in placentas collected after delivery.
Making connections
The Stanford team has been trying to boost the clock’s accuracy by adding layers of data from outside the immune system. “We established that the immune system does change throughout pregnancy and that it’s very systematic,” Aghaeepour says. “But we know that the immune system doesn’t act in isolation.”
The group recently integrated its immune measurements with several other data sources from 17 pregnant women: their gut, vaginal and oral microbiomes, blood levels of proteins and metabolism-related molecules, plus fetal genetic material released into the women’s blood. A machine learning algorithm found that the data as a whole were far more accurate at predicting gestational age than any one type alone. The study, published in January in Bioinformatics, included thousands of measurements.
Agheeapour says the more samples they can collect to teach the algorithm, the more accurate the program will be, and the better it can point researchers to the important drivers of preterm birth risk. The plan is to pare down the test to make it usable in resource-limited settings.
The Stanford team is not alone in its attempt to combine different kinds of data for one test. Instead of looking to blood, microbiologist Jennifer Fettweis of Virginia Commonwealth University in Richmond and her colleagues are putting together huge datasets from pregnant women’s vaginal microbiomes. The researchers recently tracked microbiome composition and microbe gene activity from vaginal swabs collected throughout the pregnancies of 597 women. The researchers combined that data with periodic measurements of the women’s vaginal cytokine levels.
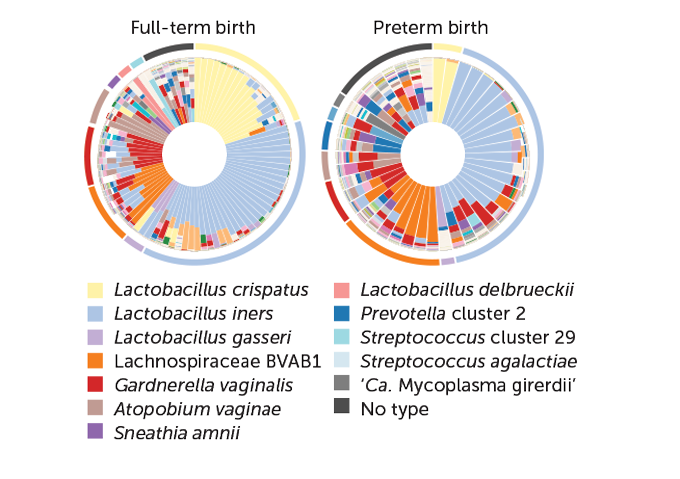
In a sample of mostly African-American women, 90 had delivered full-term and 45 had delivered preterm. The women who delivered preterm tended to have a more diverse mix of microbes than those who delivered at term, the group reported in June in Nature Medicine. The women who delivered early had much lower levels of Lactobacillus crispatus and they carried more of other types of bacteria, such as Lachnospiraceae BVAB1, that are linked to higher levels of cytokines that instigate inflammation and to vitamin D deficiency — two factors previously tied to preterm birth. The researchers suggest that microbiome changes could be a useful predictor of preterm labor risk. But because people’s microbiomes vary with geography and diet, among other things, no one microbiome profile will be predictive for everyone.
In an accompanying study, Fettweis and colleagues reported finding differences in microbiome diversity among African-American, Hispanic and white women who delivered full-term babies. The researchers don’t yet know why these differences exist, but they hope that the microbiome will hold clues about why African-American women are 1.5 times as likely to give birth prematurely and twice as likely to have very preterm infants (born before 34 weeks) as white women in the United States.
Many researchers think the microbiome differences are related to environmental factors, such as stress and nutrition. A group at Emory University in Atlanta is collecting environmental, microbiome and immune data from more than 500 pregnant African-American women to get some answers.
Drive for more data
As part of the Multi-Omic Microbiome Study-Pregnancy Initiative, Fettweis and others are analyzing microbiome samples from volunteers’ mouths, skin, vaginas and rectums, as well as blood and urine samples and health data. Fettweis would also like to explore how microbiome changes associated with preterm birth fit with other types of data. It may be that some links between the activity of certain genes or cells and preterm birth depend on the state of a mother’s microbiome, she suggests.
“We need to start thinking about these things together,” she says. “We need harmonization in the field.”
For data scientist Marina Sirota of the University of California, San Francisco, harmonizing data is a full-time occupation. She mines health data in search of relationships between risk factors and biological markers connected to preterm birth. In a meta-analysis of three studies, Sirota and collaborators found 210 genes with activity in white blood cells that differed in women who delivered preterm. Most of the affected genes were involved in immune responses, the researchers reported in 2018 in Frontiers in Immunology.
In a separate study, published in 2018 in Environment International, Sirota and colleagues matched California birth records with state data on environmental pollutants. Premature labor was more common among women living in areas with high levels of two drinking water contaminants, arsenic and nitrate. Sirota says many of the genes known to be affected by arsenic exposure are the same genes that she and colleagues found to be affected in their meta-analysis.
Work like Sirota’s might eventually point to treatment options to reduce the risk of preterm birth related to environmental exposures. After all, Gaudilliere says, the end goal is to do more than find new risk factors; the aim is to come up with therapies that target the different causes of preterm labor.
In Montreal, Chemtob has been working on a way to block the cytokine interleukin-1, which has been linked to preterm labor. On February 13 in Frontiers in Chemistry, he and colleagues described an IL-1 inhibitor that prevents inflammation-induced preterm birth in mice without hindering the cytokine’s normal anti-infection activities. The next step, Chemtob says, is lab studies testing women’s white blood cells to find the population of women who will most likely benefit.
He’s working with Gaudilliere’s group to develop a lab test that can identify women whose white blood cells change their activity when exposed to the inhibitor. The researchers plan to pair the results of the blood test with the other measurements to ultimately design a test that predicts preterm birth risk and potential drug response at the same time. “That makes for ideal personalized medicine,” Chemtob says.
“We are at the beginning of an exciting period,” says Romero at Wayne State. The field is now equipped to start studying preterm birth as a collection of several different syndromes and seek out treatments to address each one, he says.
This shift could not come soon enough. For the youngest infants, even one extra day in the womb can make a huge difference for their health.
After spending the last seven years speaking to and advocating for parents of preemies, and dealing with her daughter’s lingering health problems, Degl can attest that new ways to prevent prematurity are needed. If there was anything she could have done to extend her own pregnancy, she says, she would have done it without hesitation. “I think every mom would say that.”