By squeezing a sample of quartz to pressures higher than those deep within Earth while zapping the material with a laser, scientists have created an exotic mineral previously unknown on Earth. They speculate that it may occur naturally on some large planets.
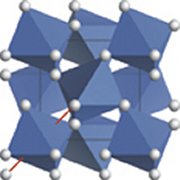
Silicon dioxide, or silica, is one of Earth’s most common chemical compounds. It makes up more than 60 percent of the planet’s crust. The substance is also one of nature’s most diverse. Its atoms aggregate in forms as common as quartz crystals and as exotic as coesite and stishovite, minerals formed by the intense pressures generated when extraterrestrial objects such as comets and asteroids strike Earth’s surface (SN: 6/15/02, p. 378).
In all, there are at least seven naturally occurring crystalline forms of silica on Earth, says Kei Hirose of the Tokyo Institute of Technology.
Now, scientists report yet another form. Hirose and his colleagues took a mixture of quartz crystals and silica glass and compressed it between two small diamonds to pressures approaching 3 million times the pressure exerted by the atmosphere at sea level. They also heated the sample with a laser to temperatures of up to 1,700°C. The diffraction pattern of X rays fired through the material provided information about the arrangements of atoms in the silica.
At low pressures, six oxygen atoms surround each silicon atom in a silica crystal. In their experiments, Hirose and his team noted that silica’s atomic arrangement became more compact at temperatures above 1,525°C and pressures above 2.6 million atmospheres. This version of silica is at least 5 percent denser than any known low-pressure form. In the dense configuration—predicted years ago but never before synthesized—each silicon atom has eight neighboring oxygen atoms. The researchers describe their feat in the Aug. 5 Science.
The new research is “impressive,” says Ho-kwang Mao of the Carnegie Institution of Washington (D.C.). Although many scientists have conducted tests at high pressures or high temperatures, “this could very well be the highest combination of pressure and temperature” ever reached in experiments on any mineral, he notes.
The newly produced type of silica probably doesn’t exist on Earth. About 2,900 kilometers below Earth’s surface, at the boundary between the outer core of molten iron and the mantle of overlying minerals, pressures measure only 1.3 million atmospheres. Most scientists speculate that below that core-mantle boundary, where pressures are even higher, there’s no silica.
Hirose’s group notes that its new form of silica might exist on large planets, such as Uranus, Neptune, or some of those discovered around distant suns. There, thick atmospheres and massive, rocky cores that likely include silica may exert the immense pressures that could make up the new mineral.
Correction: This article stated that “silica” makes up more than 60 percent of Earth’s crust, but it takes silica, which is silicon dioxide, and related silicate minerals to amount to that proportion.